Anatomy and Physiology: Chapter 2 section 4 Organic Compounds
1/108
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
109 Terms
Functional Groups of Organic Compounds
Attached groupings of atoms that occur commonly in
many organic molecules
- greatly influence the chemical (acid or base) and physical (solubility) properties of any molecule in which they occur.
4 Important Functional Groups of Organic Compounds
1 - Amino group, -NH2
2 - Carboxyl group, -COOH
3 - Hydroxyl group, -OH
4 - Phosphate group, -PO4^2-
Amino group, -NH2 (Functional group) importance
Acts as a base, because it can accept hydrogen ions (H+), depending on pH; can form bonds with other molecules
- Example: Amino acids
Carboxyl group, -COOH (Functional group) importance
Acts as an acid, because it releases a hydrogen ions (H+) to become R–COO–
- Example:
• Fatty acids
• Amino acids
Hydroxyl group, -OH (Functional group) importance
May link molecules through dehydration synthesis; hydrogen bonding between hydroxyl groups and water
molecules affects solubility
- Example:
• Carbohydrates
• Fatty acids
• Amino acids
• Alcohols
Phosphate group, -PO4^2- (Functional group) importance
May link other molecules to form larger structures; may
store energy
- Example:
• Phospholipids
• Nucleic acids
• High-energy compounds
Carbohydrate (organic molecule)
A monomer meaning "Hydrated Carbon" which is an organic molecule that contains
• carbon, hydrogen, and oxygen in ratio near 1:2:1
- Hint: C + H2O
• Referred to as saccharides (sugars)
•Monosaccharides are the building blocks of Carbohydrate
• Roughly 1.5 percent of total body weight
• Primary components of bread, pasta and lollipos
• Exmples: sugars and starches
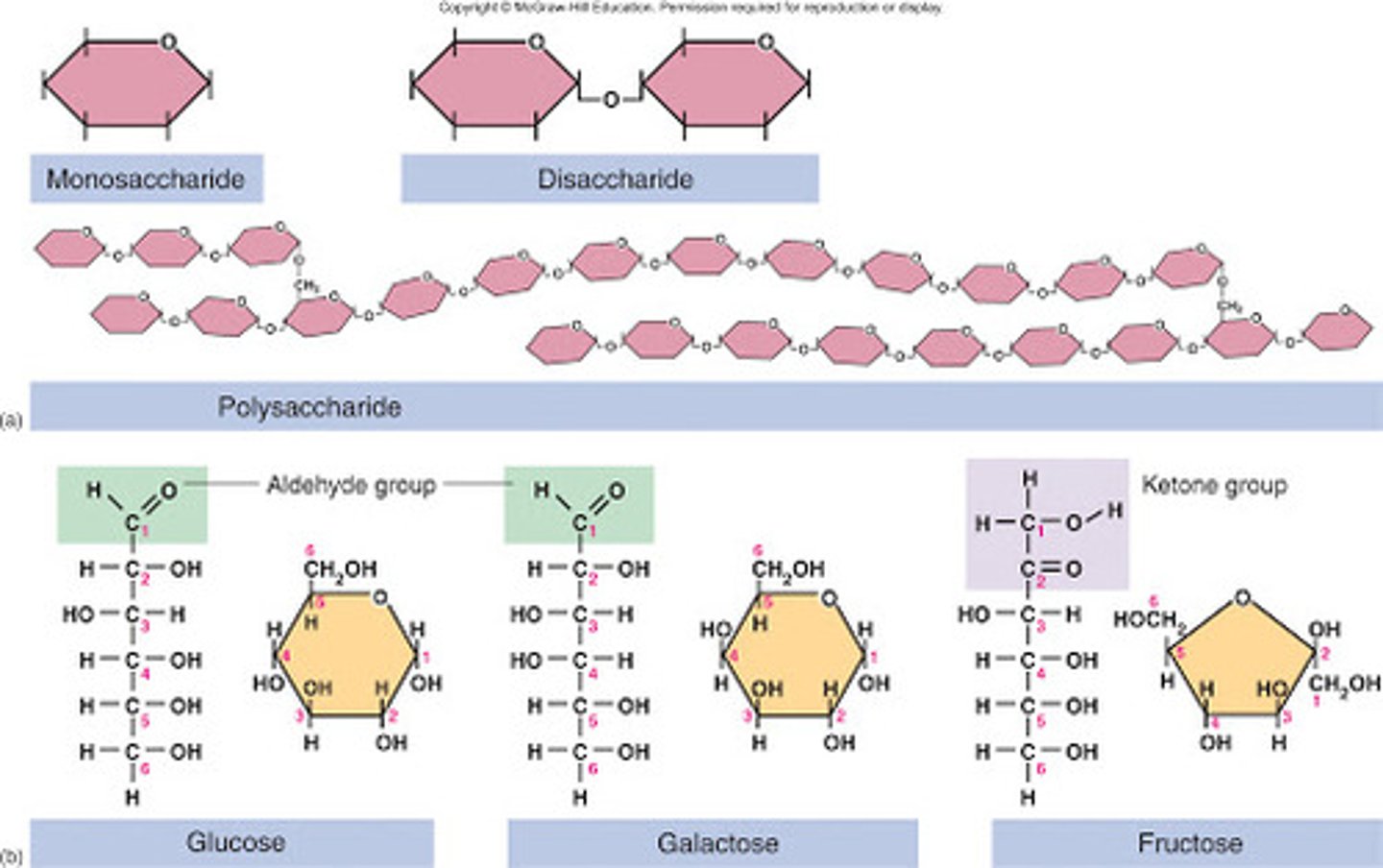
Carbohydrates importance
Main source of energy for the body that are catabolized rather than stored and covers cell membranes
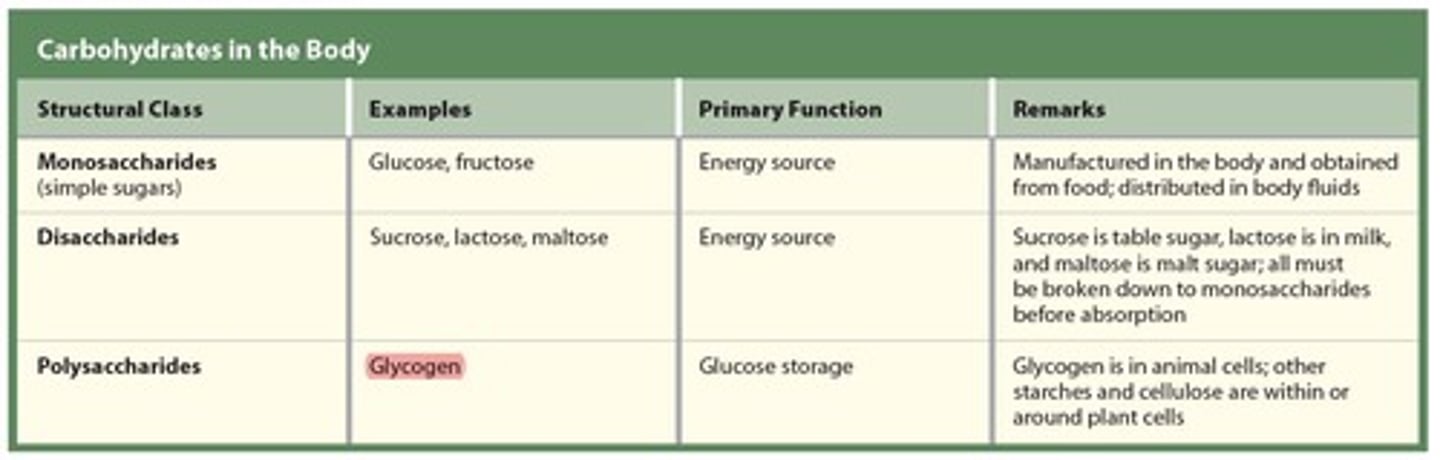
3 types of Carbohydrates in the body
1 - Monosaccharides (simple sugar)
2 - Disaccharides (two sugars)
3 - Polysaccharides (many sugars)
Monosaccharide
Is a simple/single (1) sugar that contains 3 - 7 carbons in a straight chain or a ring. used as an energy source
- the building blocks for carbohydrates.
- Manufactured in the body and obtained from food; distributed in body fluids
- can be called
triose (three-carbon),
tetrose (four-carbon),
pentose (five-carbon),
hexose (six-carbon),
heptose (seven-carbon).
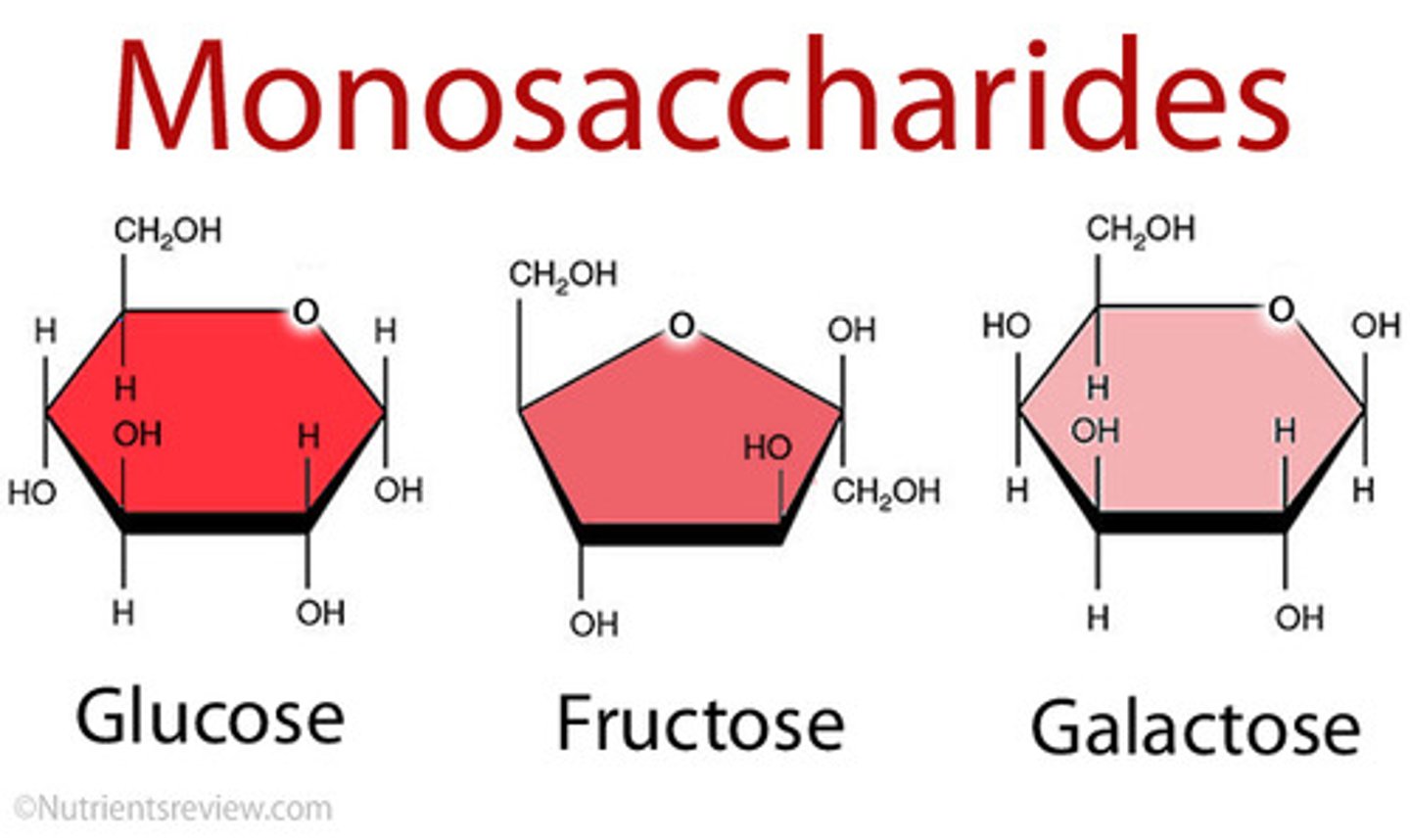
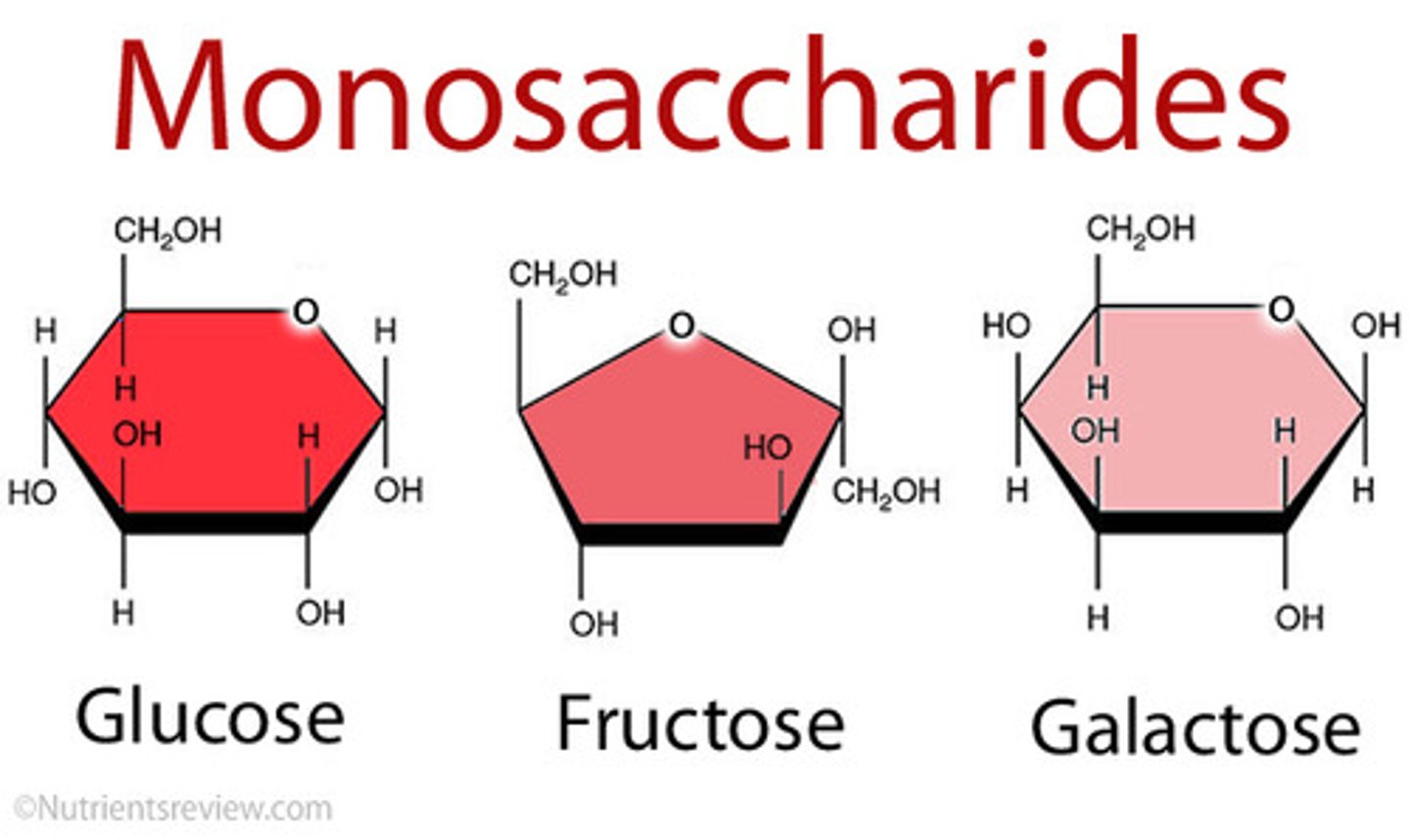
3 Examples of Monosaccharides
1 - Glucose
2 - Fructose
3 - Galactose
Glucose (monosaccharide)
A hexose (six-carbon), is a SIMPLE sugar, the primary monosaccharide and the body's main source of energy (most important metabolic "fuel")
Fructose (monosaccharide)
A hexose (six-carbon), is a FRUIT sugar (energy source)
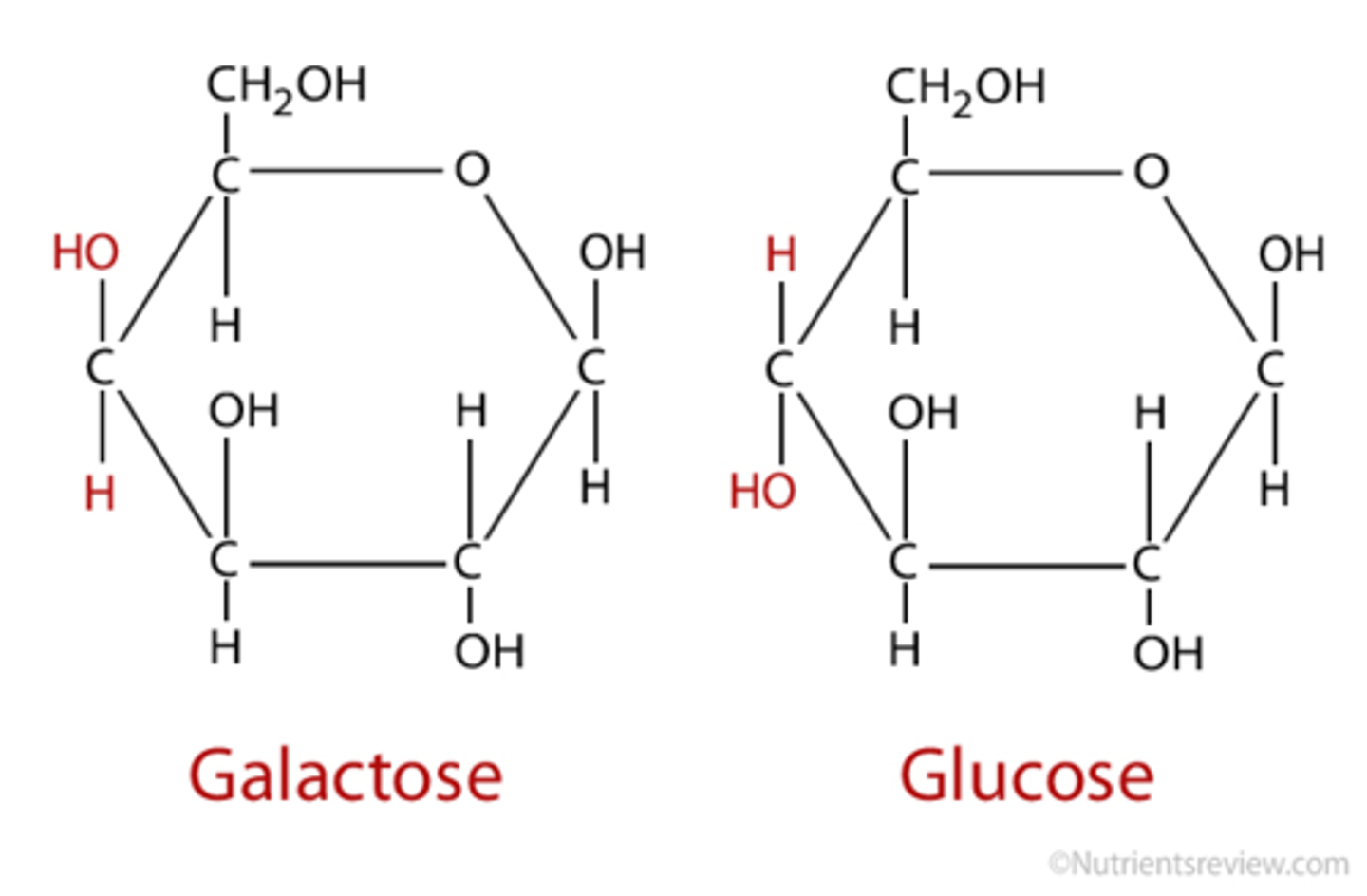
Galactose (monosaccharide)
A hexose (six-carbon), is a MILK sugar (energy source)
Monosaccharide primary function
Used as an energy source
- Manufactured in the body and obtained from food; distributed in body fluids
Isomers
Molecules that have the same molecular formula but different structures
- in other words, the same types and numbers of atoms but different structures.
Such as the monosaccharides (Glucose, Fructose and Galactose)
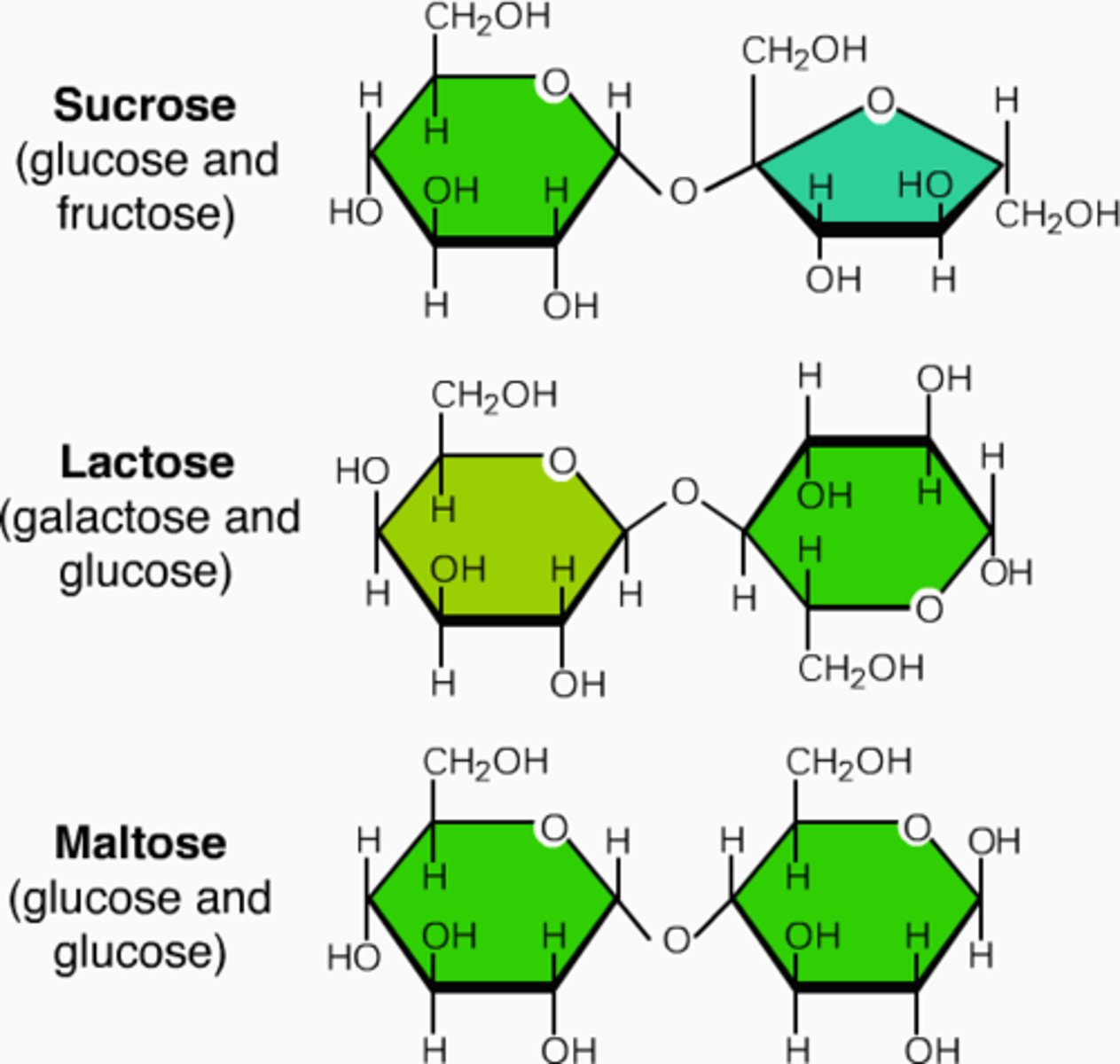
Disaccharide
* A pair of monosaccharides (It's double sugars (two)
- involves dehydration synthesis (2 molecules are joined by the removal of a water molecule.)
- Used for energy
* Examples
- sucrose (glucose + sucrose) – table sugar
- lactose (galactose + glucose) – milk sugar
- maltose (glucose + glucose) – malt sugar
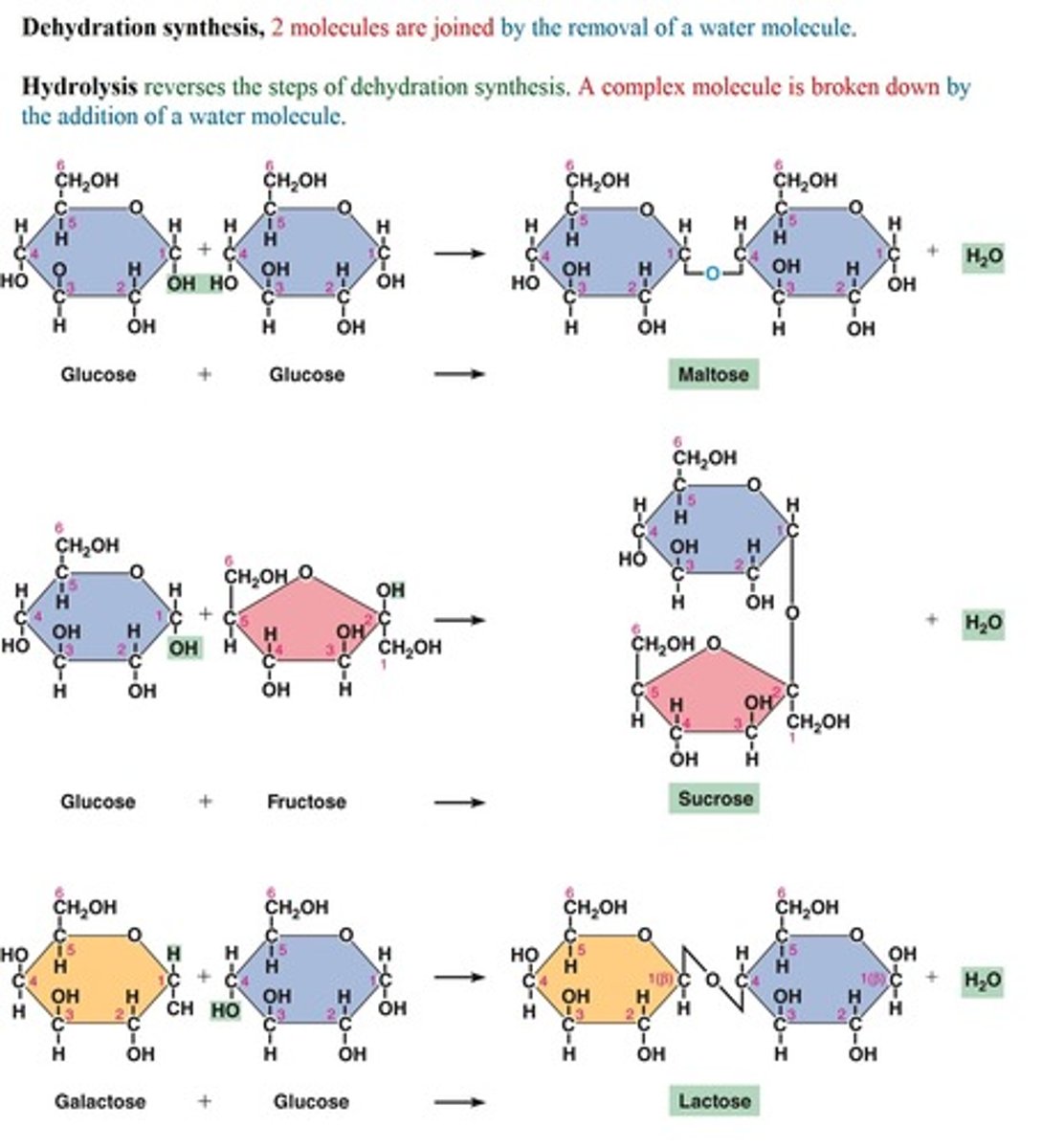
Disaccharides primary function
Used as an energy
3 Examples of Disaccharide
1 - sucrose (glucose + sucrose) - table sugar
2 - lactose (galactose + glucose) - milk sugar
3 - maltose (glucose + glucose) - malt sugar
* all 3 disaccharides must be broken down to monosaccharides before absorption
Polysaccharide
Contain a few to a thousand or more (long chain) monosaccharides composed of glucose, starches are the largest polysaccharides formed from glucose.
- Used for glucose storage
* Examples
- Glycogen – a storage form of glucose in the human liver and muscles.
- Starches – an energy source obtained from plants (Potatoes, Grains, Peas)
- Cellulose – a structural polysaccharide in plants; when consumed, it acts as a dietary fiber (from the cell wall of green plants)
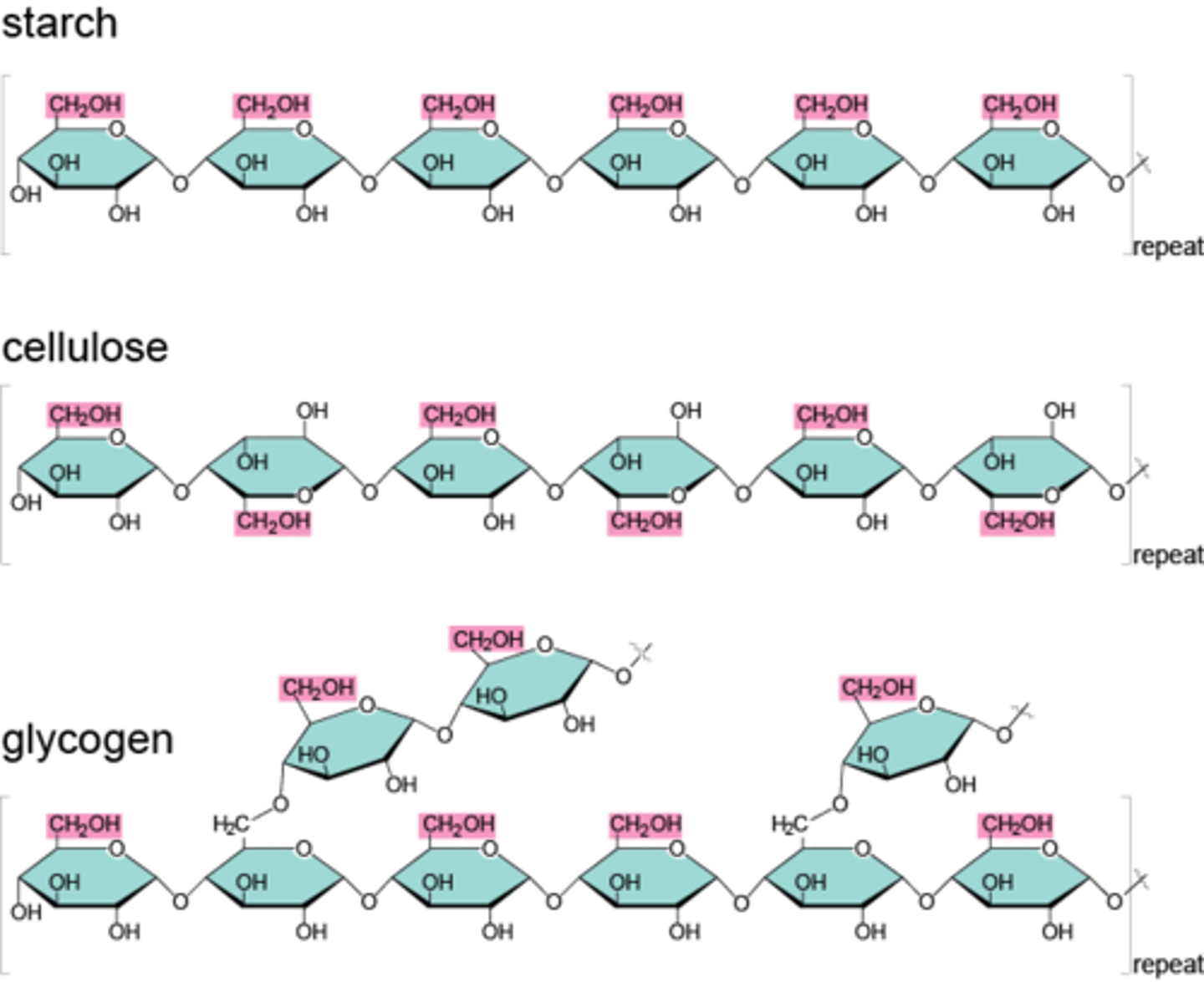
Polysaccharides primary function
Glucose storage
3 examples of polysaccharides
- Glycogen – a storage form of glucose in the human liver and muscles.
- Starches – an energy source obtained from plants (Potatoes, Grains, Peas)
- Cellulose – a structural polysaccharide in plants; when consumed, it acts as a dietary fiber (from the cell wall of green plants)
Lipid (organic molecule)
Similar to Carbohydrates, they are made up of hydrocarbons (H-C-H) called fatty acids (monomer for lipids)
- Contains mostly Carbon and Hydrogen (1;2 ratio H-C-H) and very little Oxygen (less than Carbohydrates)
- Not soluble in water (Non-Polar) (special transport mechanisms for them in the blood)
- Primary components of egg yolk and peanut oil
* Examples: fats, oils and waxes
Important as energy reserves
5 types of lipids
1. Fatty acids
2. Glycerides (Triglycerides most common)
3. Eicosanoids
4. Steroids
5. Phospholipids, glycolipids
Summarize the functions of lipids in the body.
Some act as chemical messengers between cells (Steroids & Eicosanoids)
- Essential components of the plasma membranes.
- Important as energy reserves
- Provide twice as much energy as carbohydrates
- 12–18% of total body weight for men and 18–24% for women
- Humans must obtain some fatty acids from the diet
Fatty acids (lipids)
Long carbon chains with attached/bonded hydrogen
atoms, cholesterol and phospholipids are the Monomer (building block). Primarily functions as an Energy source
- It’s absorbed from food or synthesized in cells: then transported in the blood
- Two ends
1. Head
- Composed of a Carboxyl group (O-C=OH)
– Polar ( water liking, "Hydrophilic")
2. Tail (can be saturated or unsaturated)
- Composed of long Hydrocarbon chains (H-C-H)
– Non Polar (water hating, "Hydrophobic")
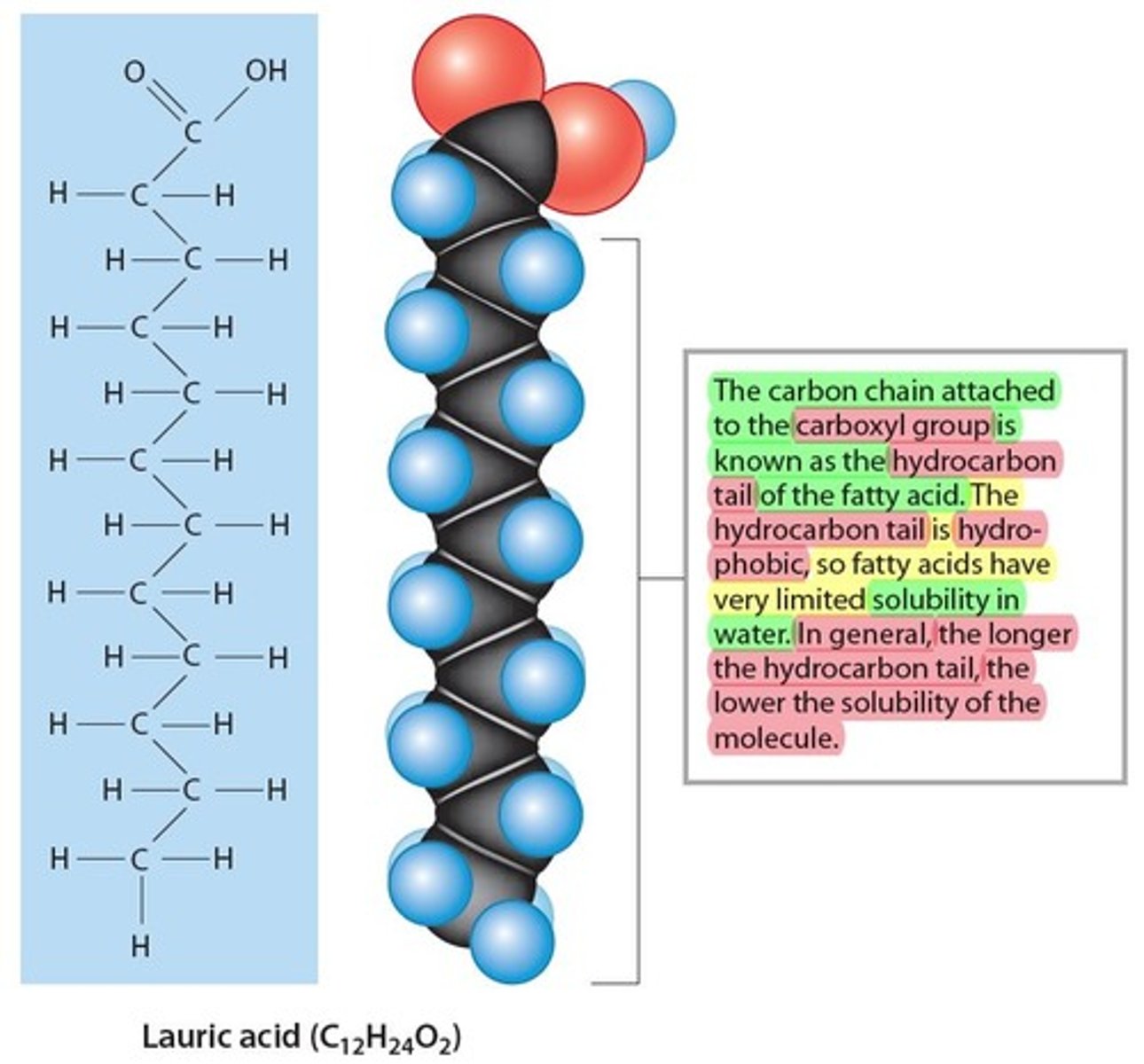
saturated fatty acids
Are in the tail of a fatty acid (Non-Polar, water hating, "hydrophobic") composed of Hydrocarbon (H-C-H)
- containing 4 single covalent bonds
- they tend to be found in butter, lard and animal products (fats)
- Not healthy for us
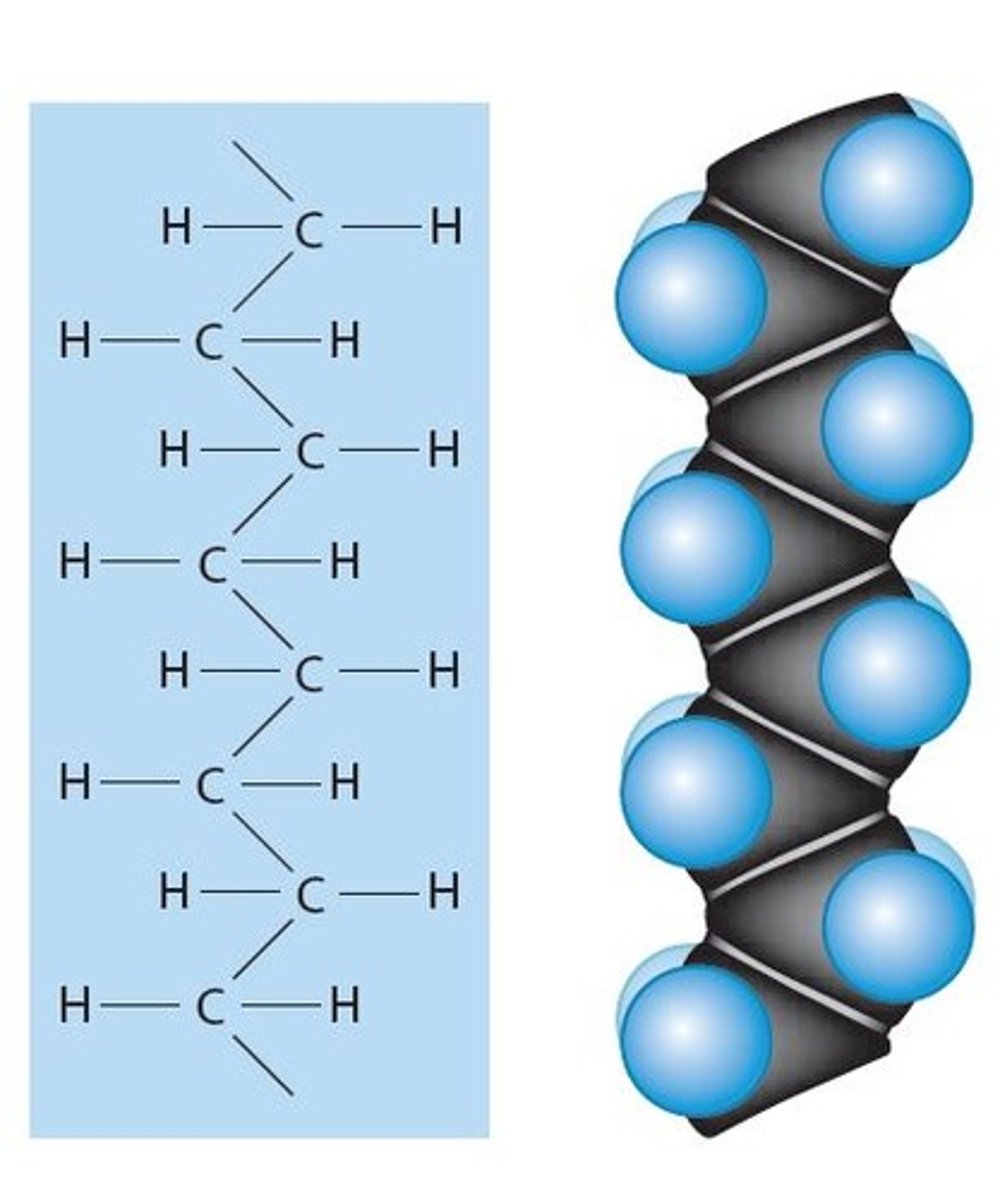
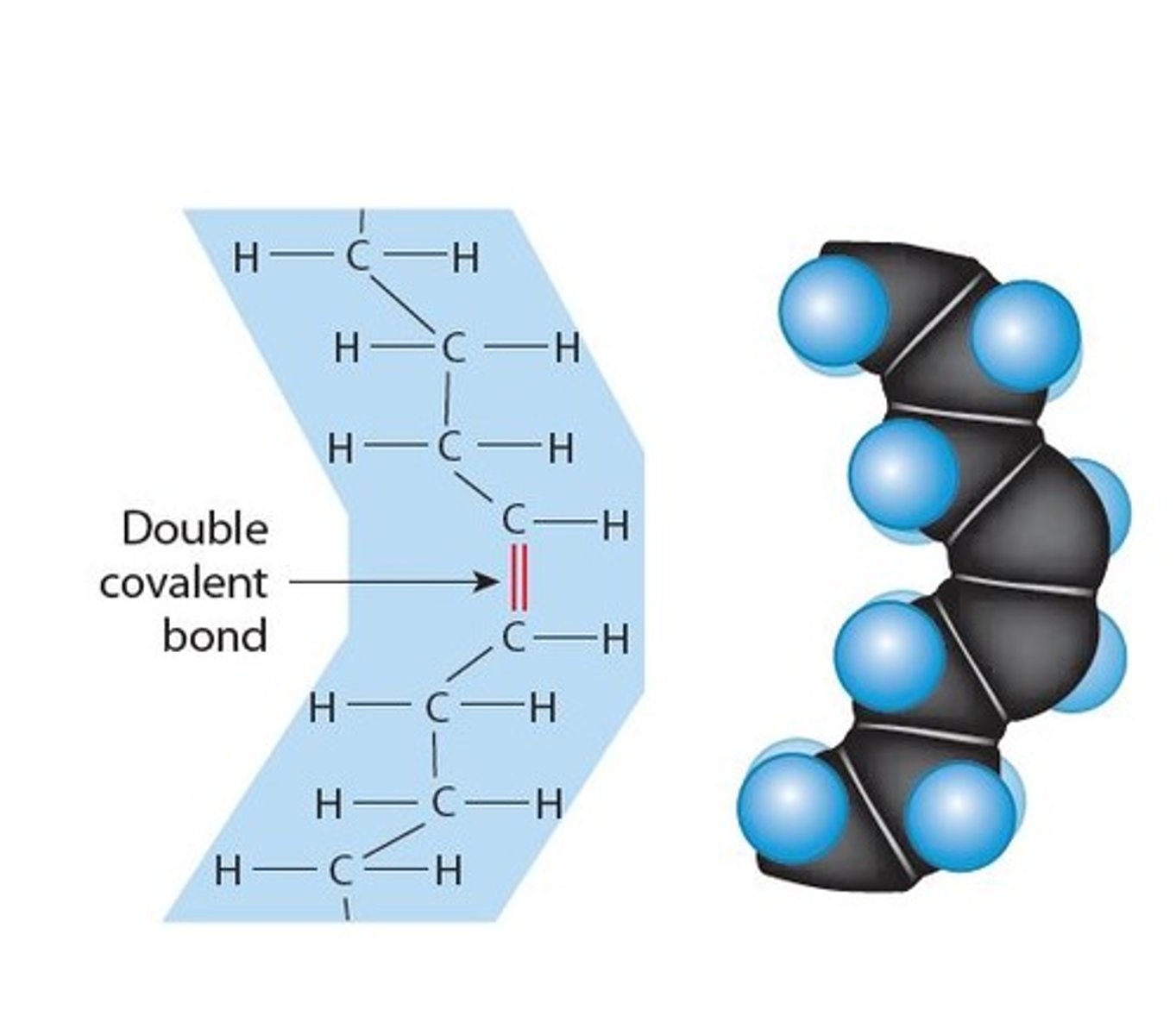
Unsaturated fatty acids
Are in the tail of a fatty acid (Non-Polar, water hating, "hydrophobic") composed of Hydrocarbon (H-C-H) containing 1 or more double covalent bonds in the hydrocarbon tail.
- A monounsaturated fatty acid has a single double covalent bond
- A polyunsaturated fatty acid contains multiple double covalent bonds
- They tend to be found in plant products (oils)
- and are healthier fats for us
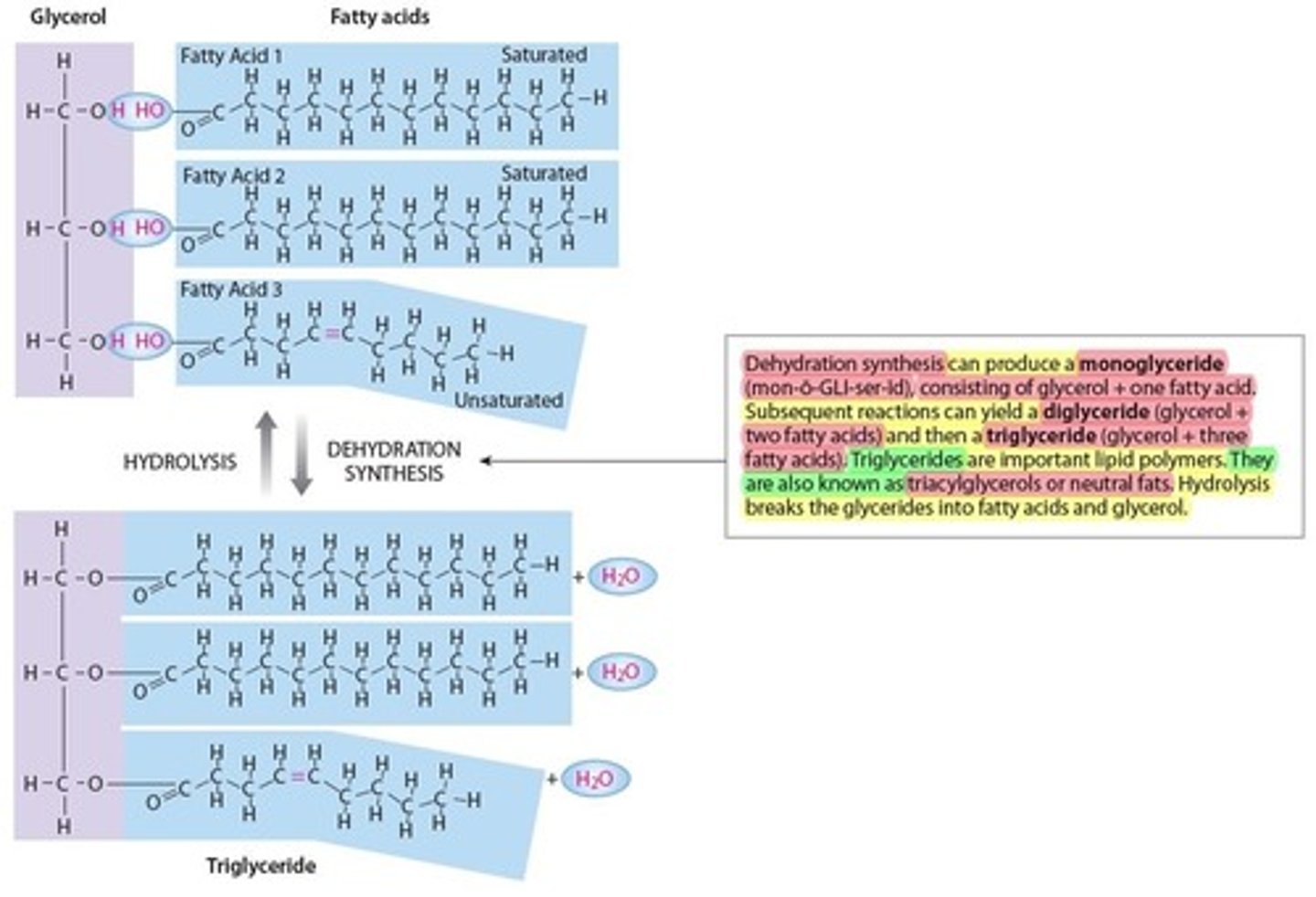
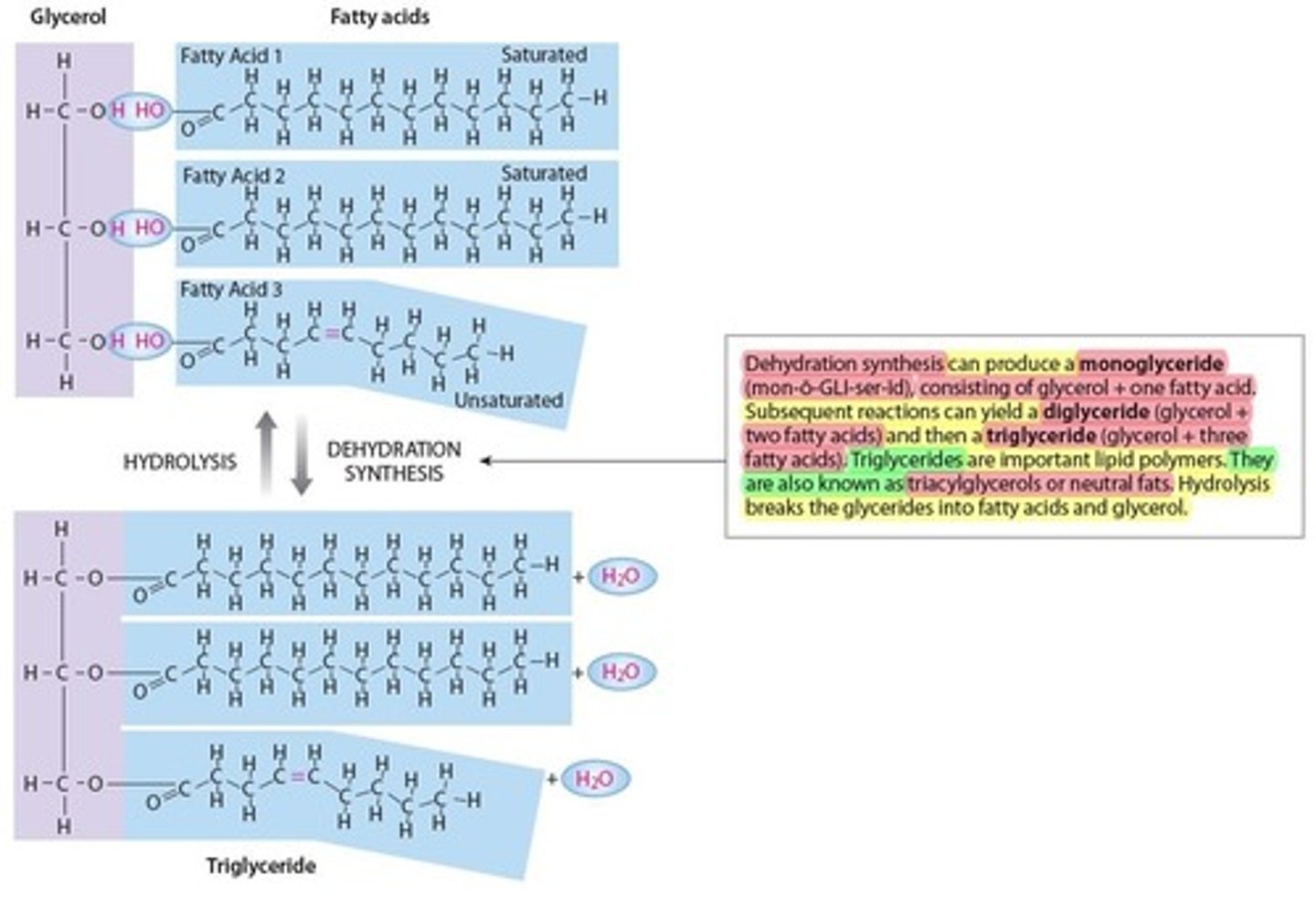
Glycerides (lipids)
Is the most common form of lipids
- Fatty acid chains attached to a glycerol molecule (bulding block of Glyceride)
- 3 Types of Glycerides
1. Monoglyceride (1 glycerol + 1 fatty acid)
2. Diglyceride (1 glycerol + 2 fatty acids)
3. Triglyceride (1 glycerol + 3 fatty acids)
- Also known as triacylglycerols or neutral fats
- adipose (fat tissue)
Glycerides 4 primary functions
1 - energy source
2 - Energy storage,
3 - Insulation and
4 - Provide a physical protection
*Stored in fat deposits; must be broken down to fatty acids and glycerol before they can be used as an energy source
3 types of Glycerides
1. Monoglyceride (1 glycerol + 1 fatty acid)
2. Diglyceride (1 glycerol + 2 fatty acids)
3. Triglyceride (1 glycerol + 3 fatty acids)
– Also known as triacylglycerols or neutral fats
– adipose (fat tissue)
Eicosanoids (lipids)
Lipids derived from arachidonic acid, a fatty acid that must be absorbed from food/diet, because it's not synthesized by the body
Eicosanoids primary function
Chemical messengers coordinating local cellular activities
2 types of Eicosanoids
1 - Leukotrienes
2 - Prostaglandins
Prostagladins
Are one type of the eicosanoid lipids derived from arachidonic acid (a fatty acid). ____________________ are released by cells to coordinate local cellular activities. They are extremely powerful even in minute quantities and also produce pain sensations when released by
damaged tissues.
Leukotrienes
Are one type of the eicosanoid produced primarily by cells involved with coordinating the responses to injury or disease
Steroids (lipids)
chemical messengers with a large molecules containing four carbon rings
• Made from cholesterol
- Functions to maintain plasma membranes
- Also needed for cell growth and division
Why is cholesterol necessary in the body?
Because it's a component of the plasma membranes and it's important for cell growth and division.
3 Type of steroid
1 - cholesterol
2 - Sex hormones (estrogen & testosterones), regulation of sexual and other metabolic functions
3 - Adrenal gland hormones (cortisol) to regulate stress
Steroid primary function
Structural components of cell membranes, hormones, digestive secretions in bile
What are the important functions of steroids?
Cells need cholesterol (a type of steroid) to maintain their plasma membranes
Steroid hormones are needed in the regulation of sex hormones
Steroid hormones are important in the regulation of tissue metabolism and mineral balance
Steroid derivatives (Bile salts) are required for the normal processing of dietary fats
Phospholipids and glycolipids (lipids)
Both are Diglycerides ( 1 glycerol + 2 fatty acids)
- Both are structural Lipids "fats" that compose Cell and Plasma Membranes
- Both derived from Fatty Acids and Non-Lipid components that contain a Hydrophobic Hydrocarbon tail (H-C-H) that is Non-Polar with water and a hydrophilic Nonlipid head that is Polar with water
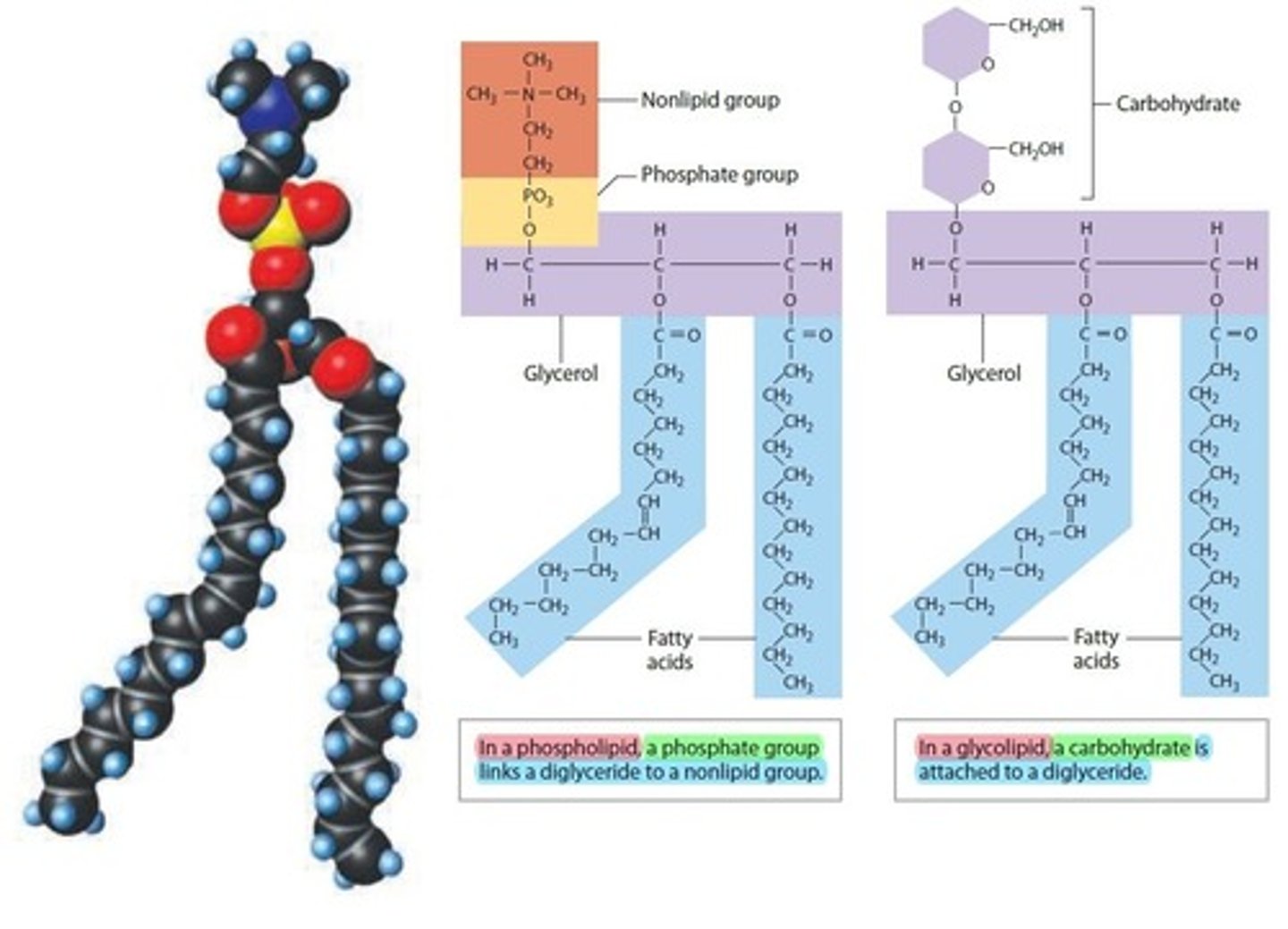
What do cholesterol, phospholipids, and glycolipids have in common?
Cholesterol, phospholipids, and glycolipids are structural lipids that form membranes of cells.
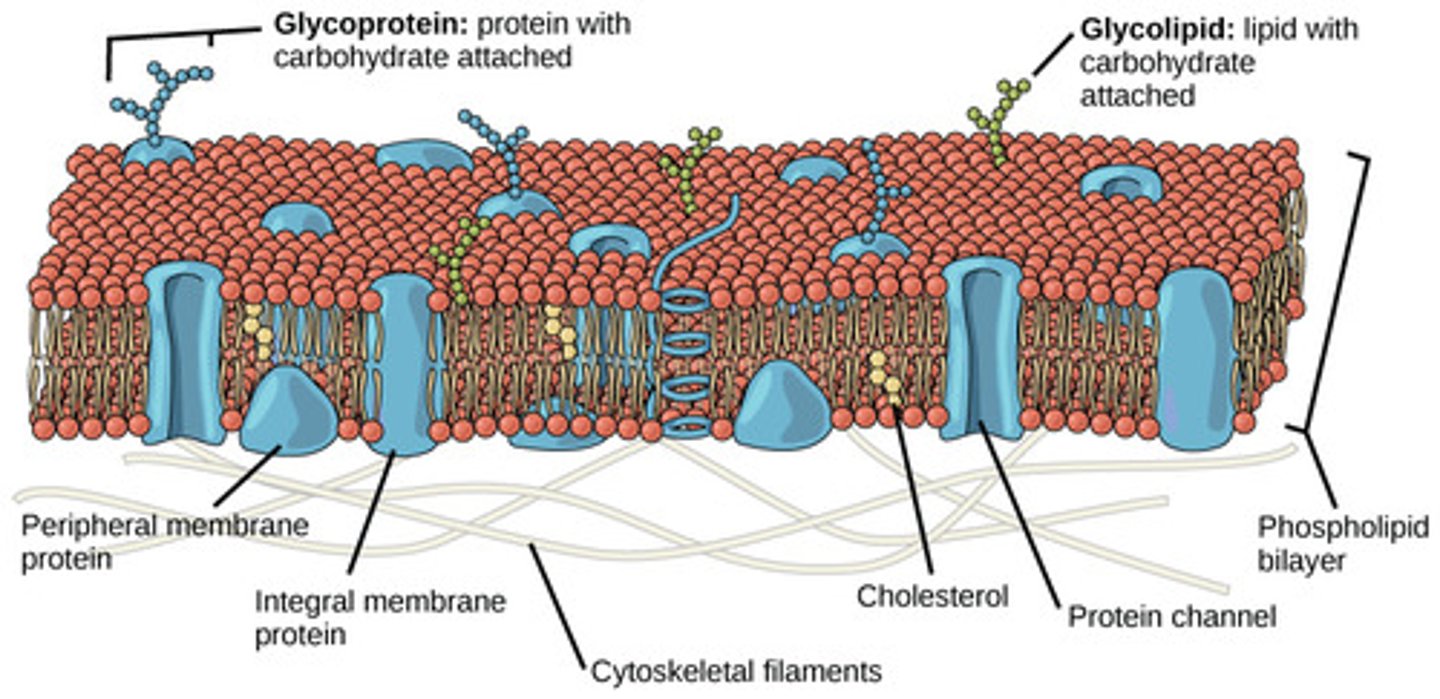
Proteins
Contain Carbon, Hydrogen, Oxygen, Nitrogen and possibly Sulfur and Phosphorus as well
• Includes collagen, hemoglobin and Keratin
• Normally account for 20 percent of total body weight
• Three-dimensional shape determines functional properties
Example: meats and cheese
Most abundant/important organic component in the human body is
Protein (Amino Acid are the monomers, building blocks)
- Consist of long chains of amino acids
• 20 different amino acids in the body
• Typical protein contains 1000 amino acids
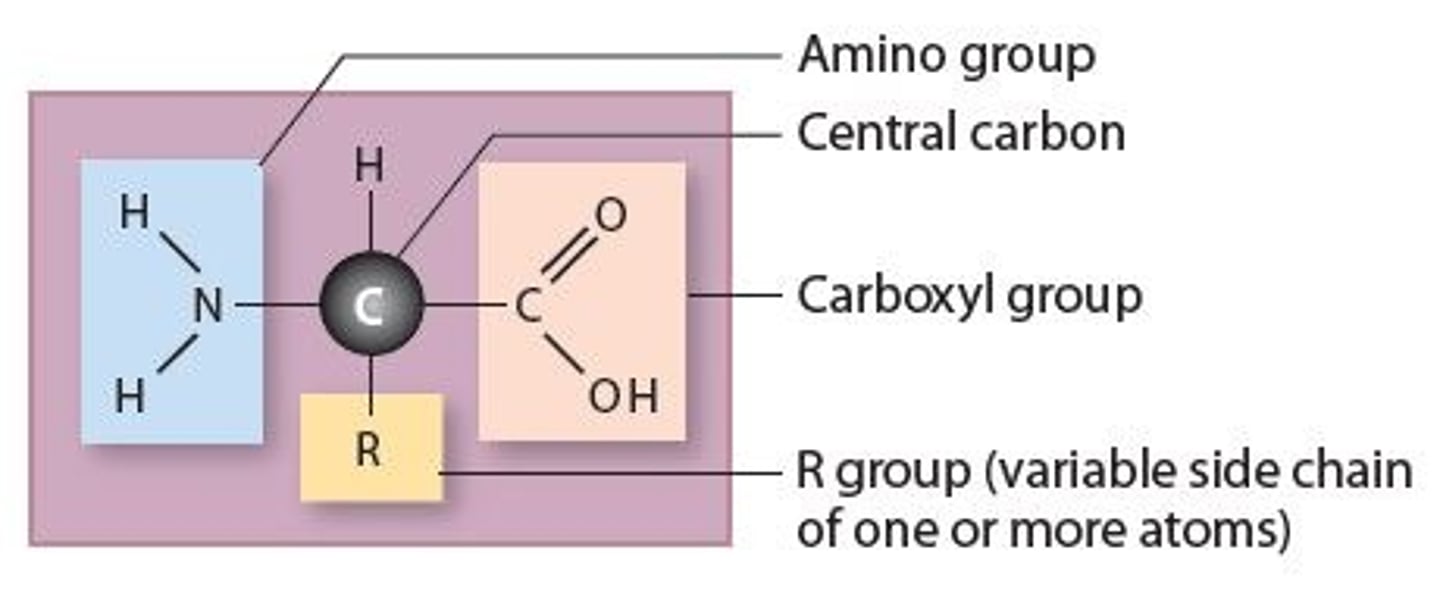
Amino acids and structure
Are simple organic monomers that form proteins. All ________________ have the same structural components that consists of a
* Central carbon attached to four different groups
1. Hydrogen atom
2. Amino group (H-N-H)
3. Carboxyl group (O=C-OH)
4. R group (variable side chain that gives amino acid chemical properties)
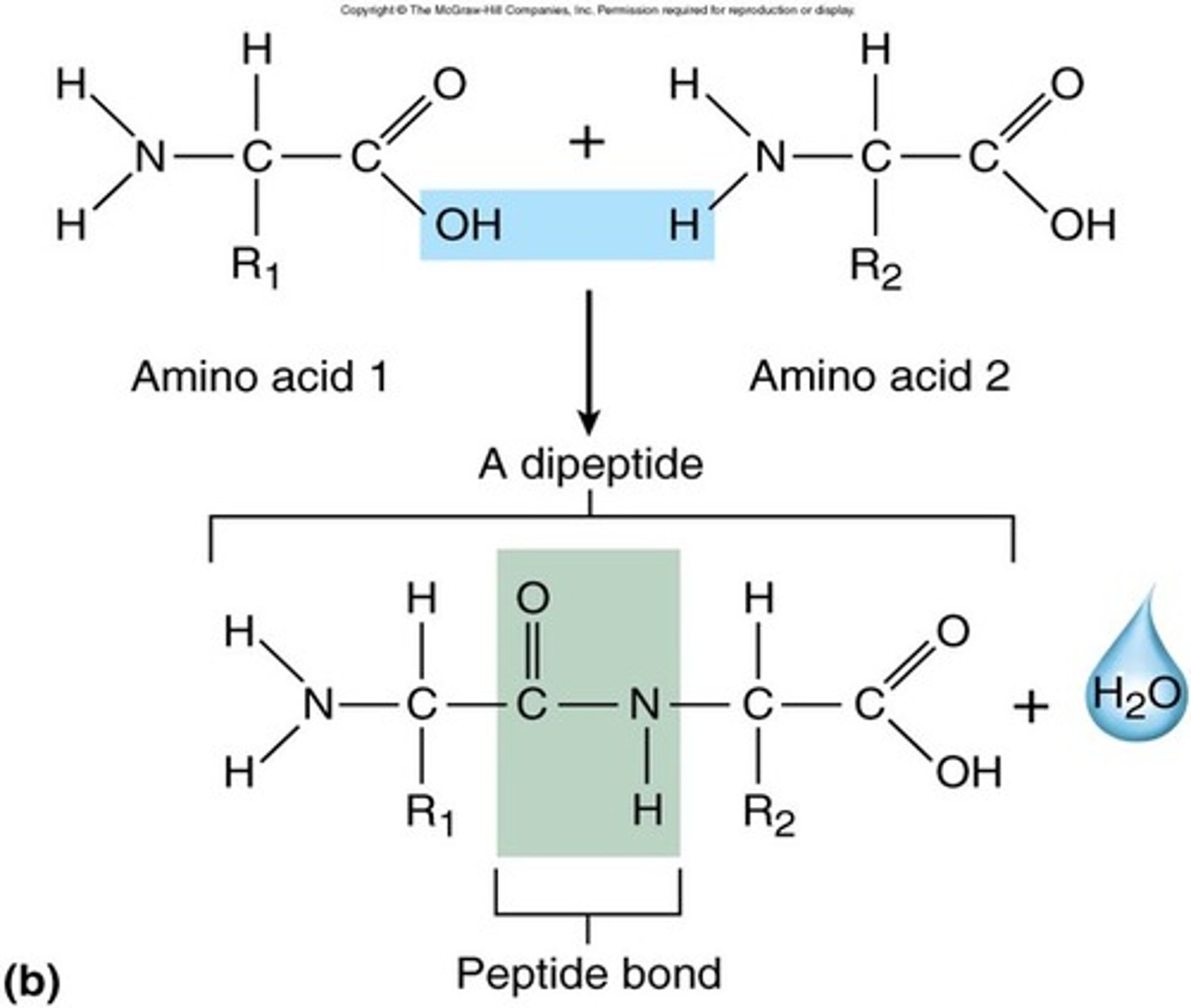
Peptide bond
A covalent bond that links two amino acids together by dehydration synthesis. (links 1 amino acids Carboxyl group (O=C-OH) to an Amino group (H-N-H) of another amino acid)
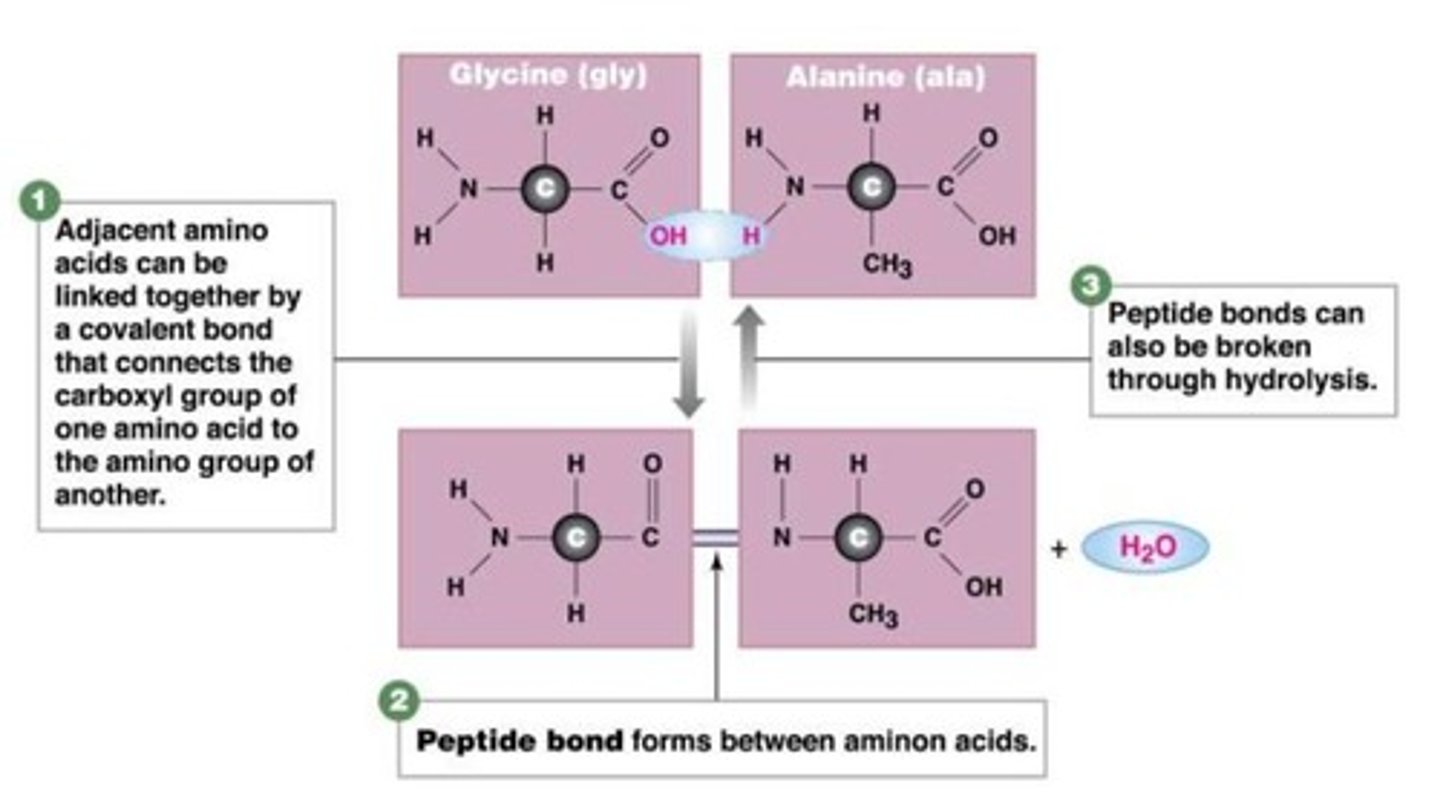
Dipeptide bond
2 amino acids bonded together
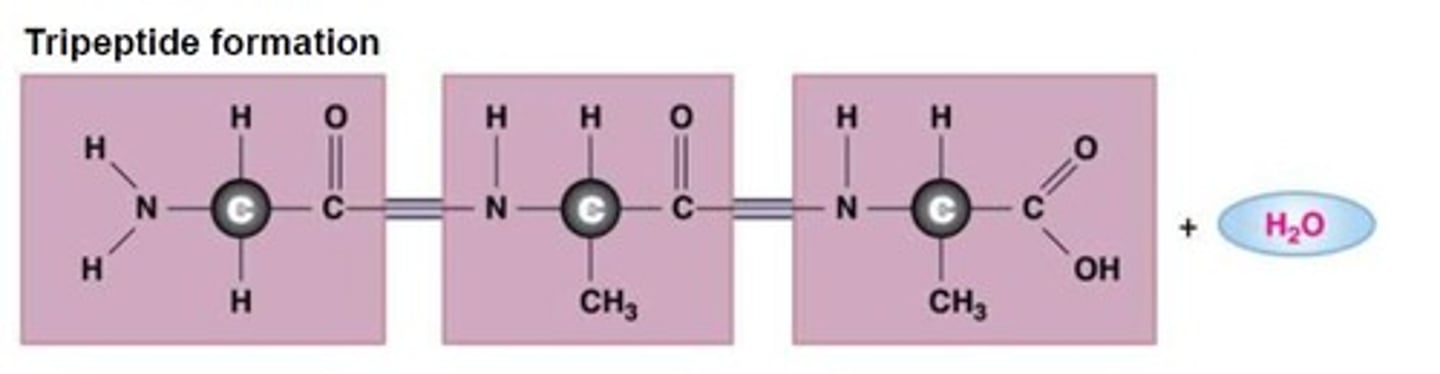
Tripeptide bond, AKA Polypeptide
3 or more amino acids linked together
Polypeptides containing more than 100 amino acids
Are called proteins
- Can have up to 4 levels of structural complexity.
1 - Primary structure
2 - Secondary structure
3 - Tertiary structure
4 - Quaternary structure
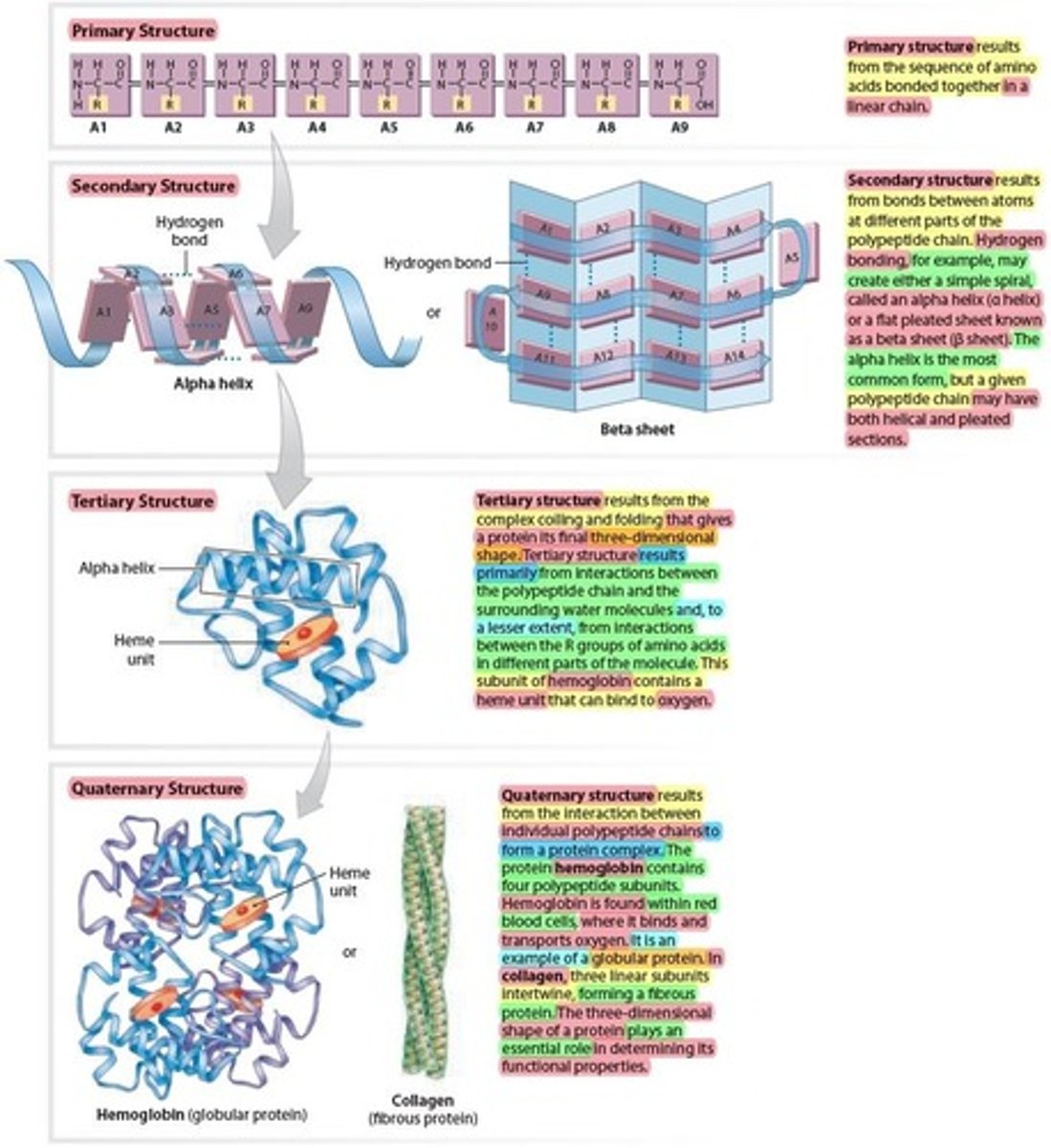
Primary structure of a Protein
Results from the sequence of amino acids bonded together in a linear chain.
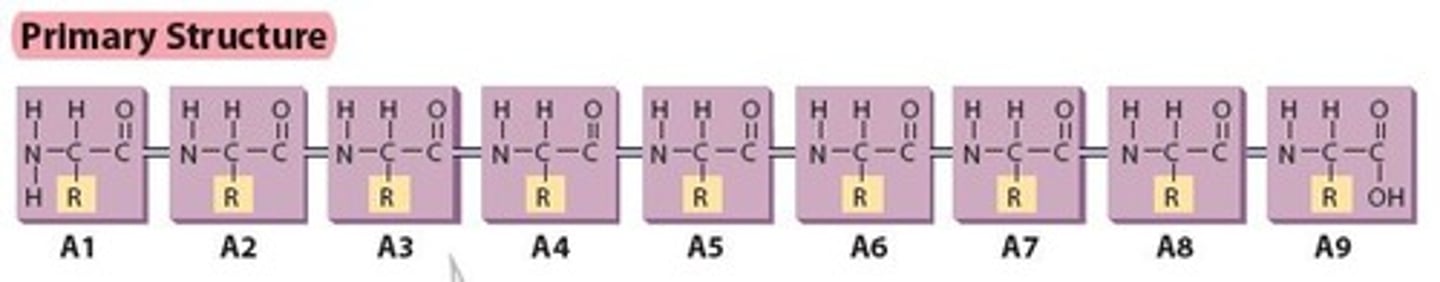
Secondary structure of a Protein
Results from bonds formed between atoms at different parts of the polypeptide chain
– Example: hydrogen bonds
• May create simple spiral (alpha-helix) or flat pleated
sheet (beta sheet)
- simple spiral (Alpha-helix) is the most common
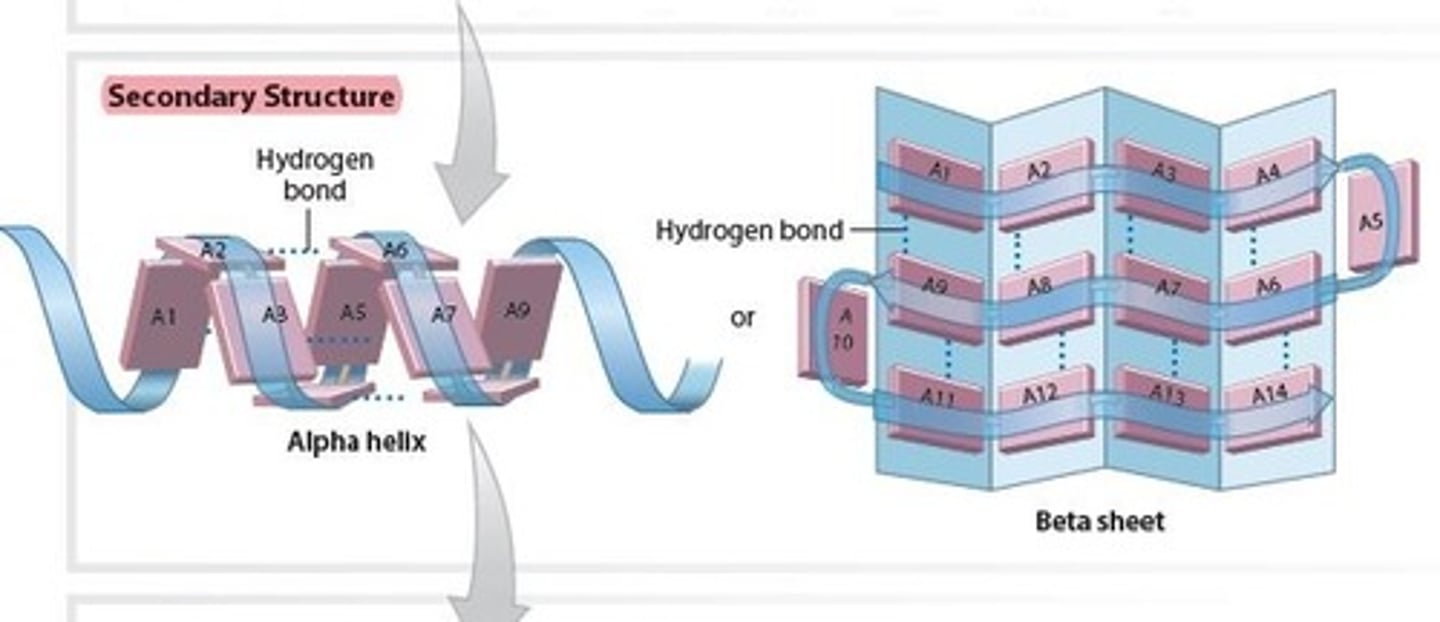
Tertiary structure of a Protein
Results from the complex coiling and folding that gives
a final 3D shape/structure to the Protein
- primarily from interactions between the polypeptide chain (Protein) and the surrounding water molecules and, to a lesser extent, from interactions between the R groups of amino acids
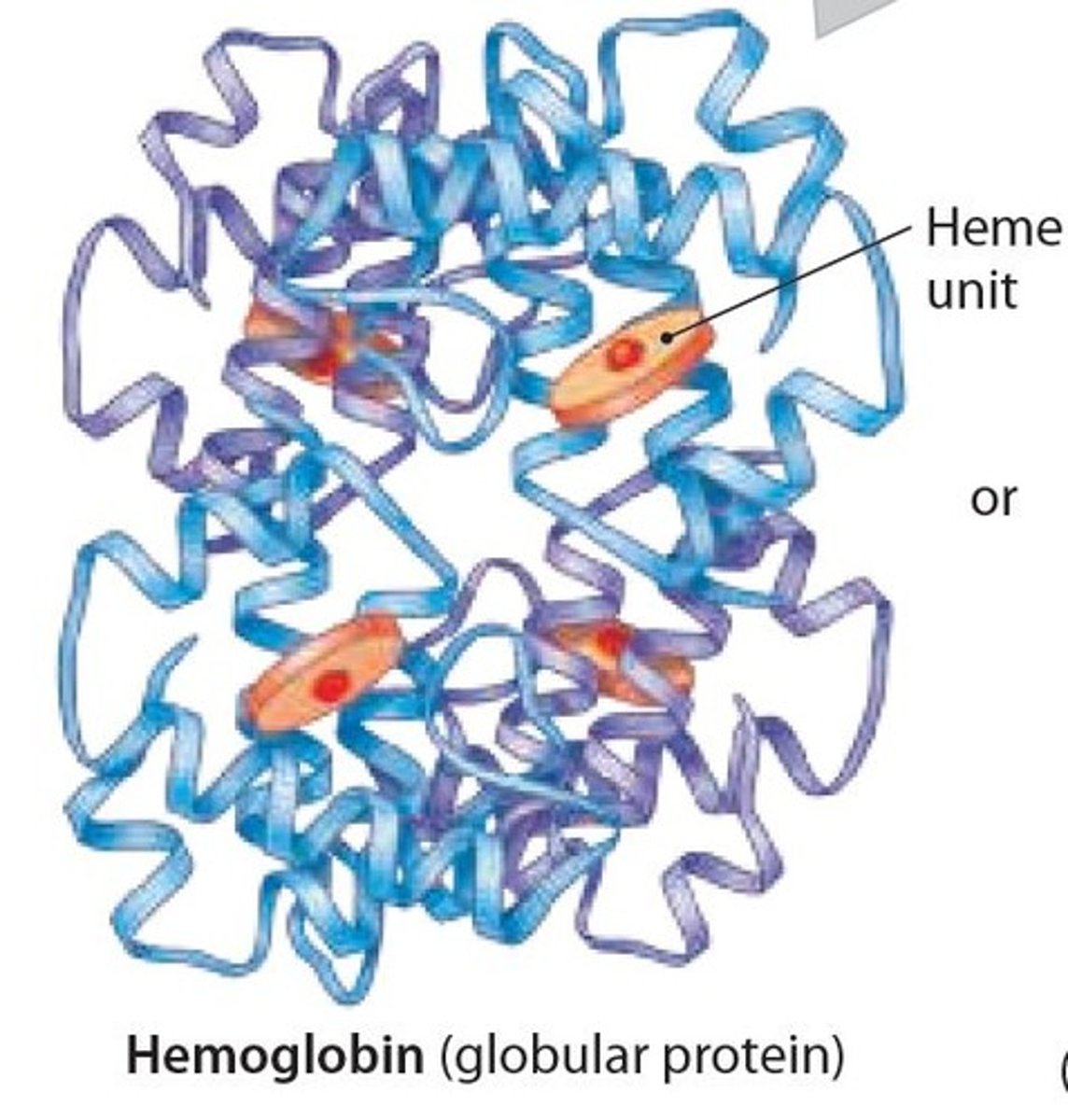
- This subunit of hemoglobin contains a Heme unit that can bind to oxygen.
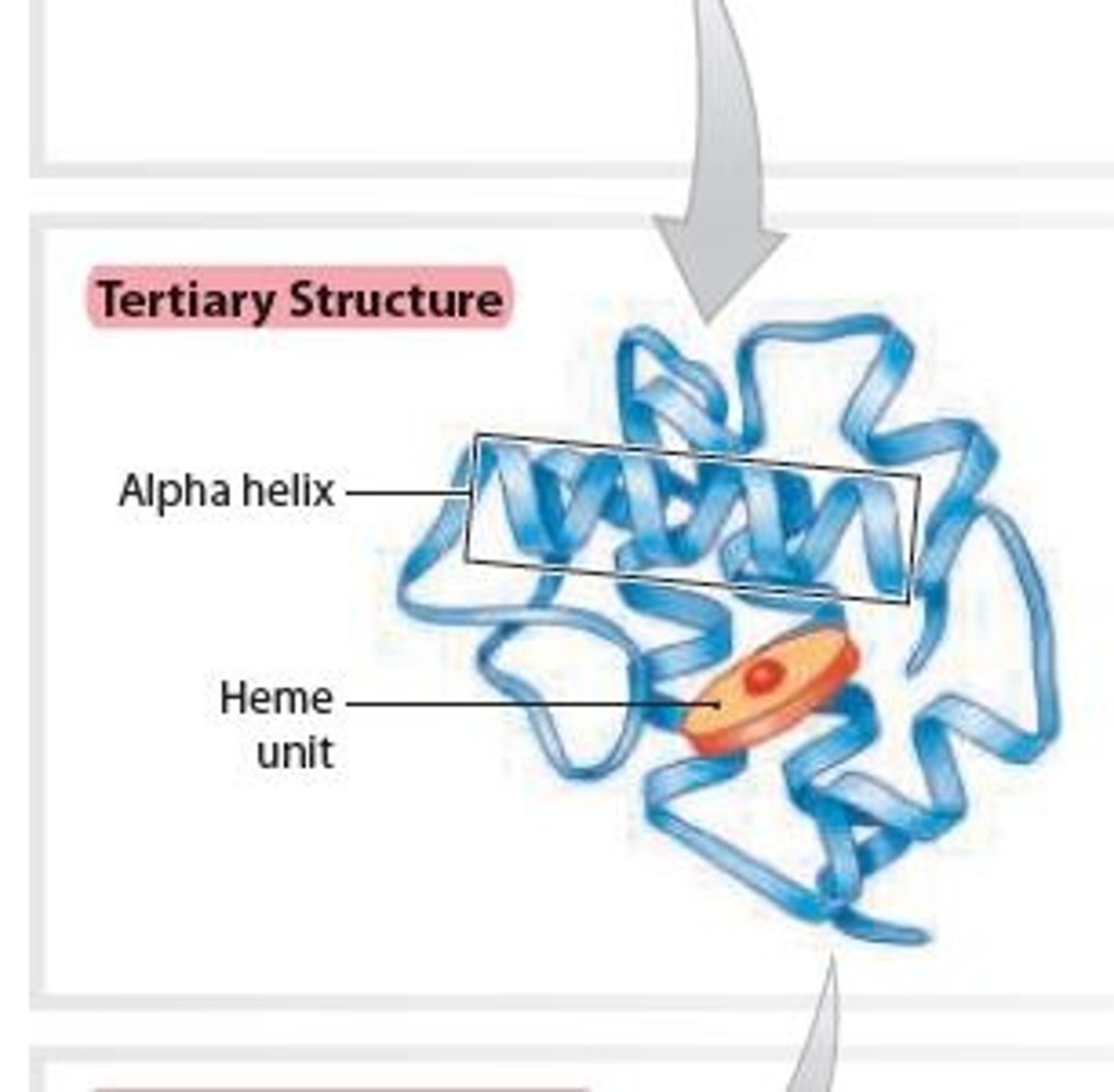
Quaternary structure of a Protein
Results from interaction between multiple polypeptide chains forming a protein complex
2 Examples:
1 • Hemoglobin
2 • Collagen
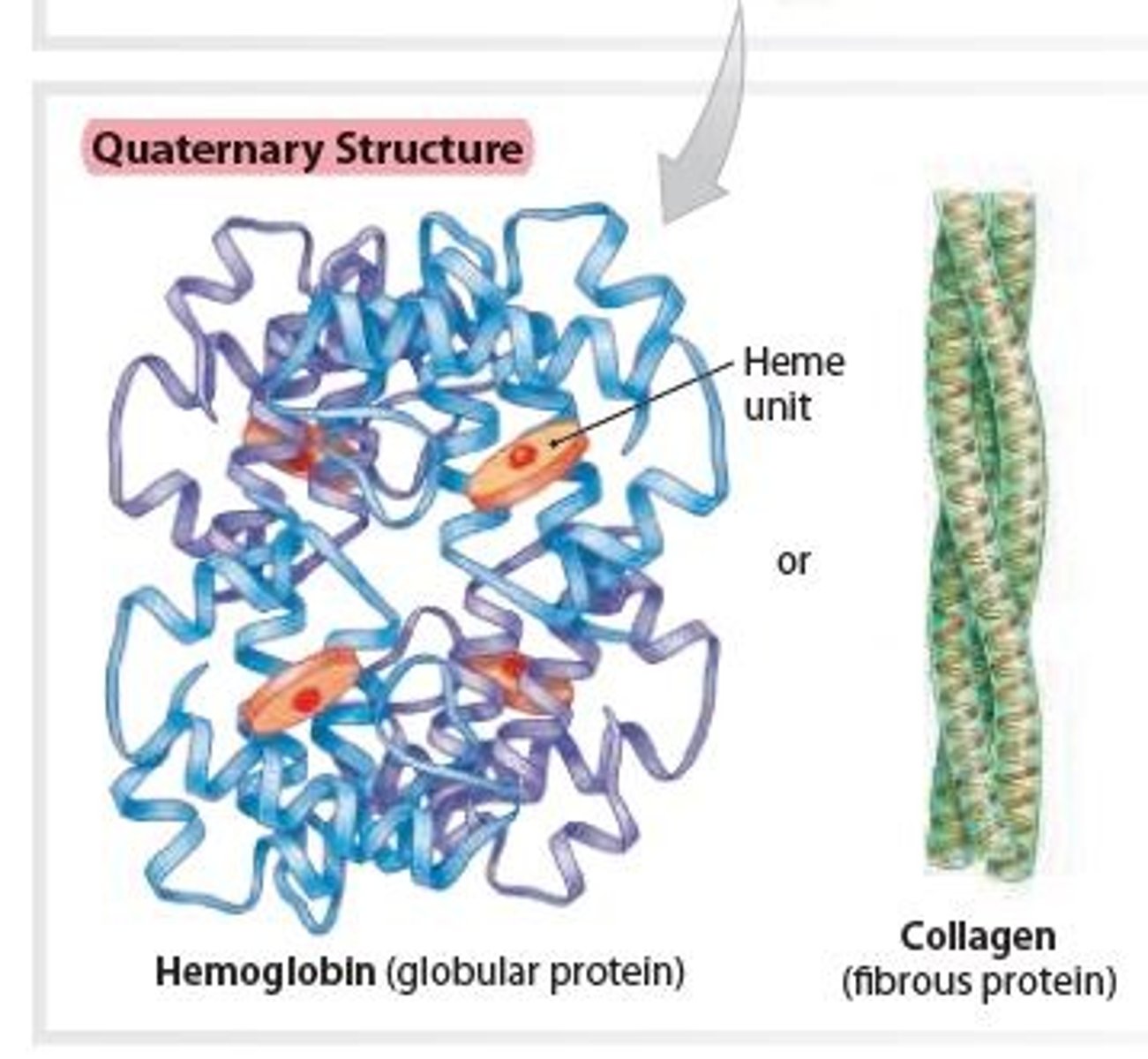
Hemoglobin in Quaternary structure of a Protein
Contains 4 polypeptide subunits (4 Heme units) that form a globular protein
– found within red blood cells, where it binds and transports oxygen.
Collagen in Quaternary structure of a Protein
- Three linear subunits intertwine, forming a fibrous
protein that give strength to tissues

What are the essential functions of proteins?
Support
Movement
Transport
Buffering
Metabolic regulation
Coordination and control
Defense
What distinguishes on amino acid from another?
The different R groups
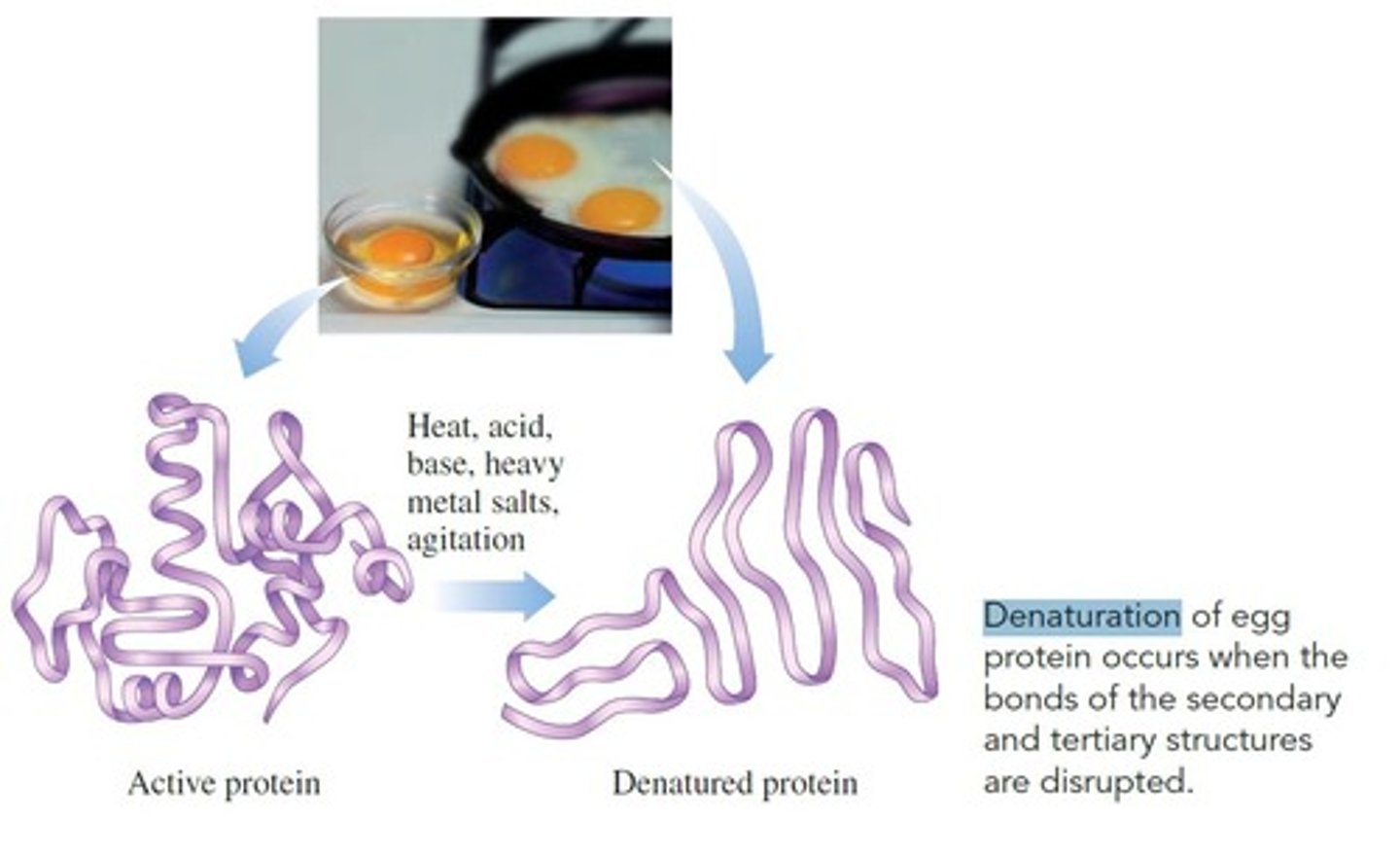
Denaturation of a Protein
Protein unfolding/looses its 3D shape and function deteriorates (does not work anymore). Death occurs when the body temperature rises above 43ºC or 110ºF, which causes irreversible damage to organs and organ systems, due to the _________________ of structural proteins and enzymes
What kind of bond forms during the dehydration synthesis of two amino acids, and which functional groups are involved?
During the ____________________ synthesis of two amino acids, a peptide bond is forms to link 2 functions groups, the amino group of one amino acid bonds with the carboxyl group of the other amino acid.
Why does boiling a protein affect its functional properties?
Because of Denaturation. As temperatures rise, protein
shape changes and enzyme function deteriorates. Eventually the protein undergoes denaturation, which affects the ability of the protein molecule to perform its normal biological functions.
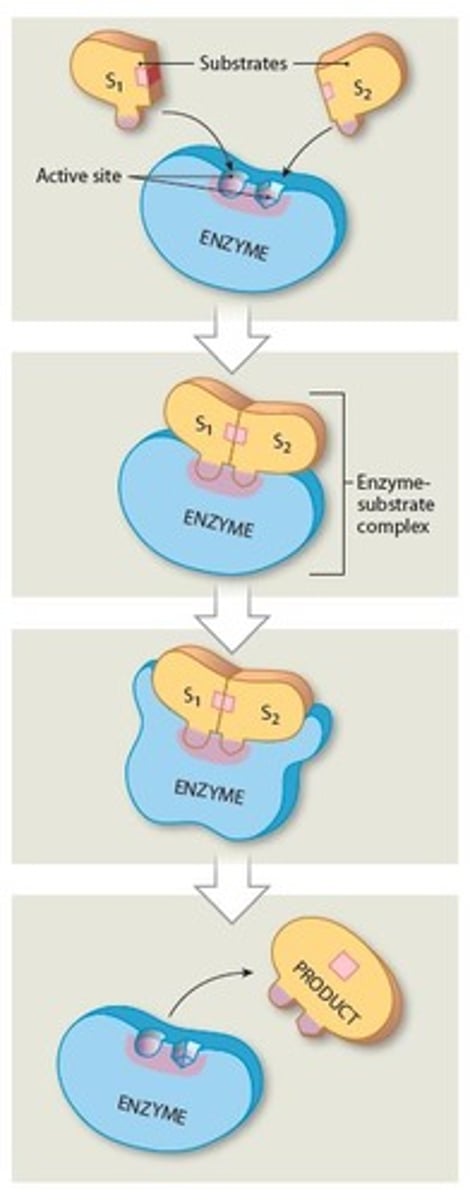
Substrates
Reactants in enzymatic reactions
- Interactions among substrates yield specific products
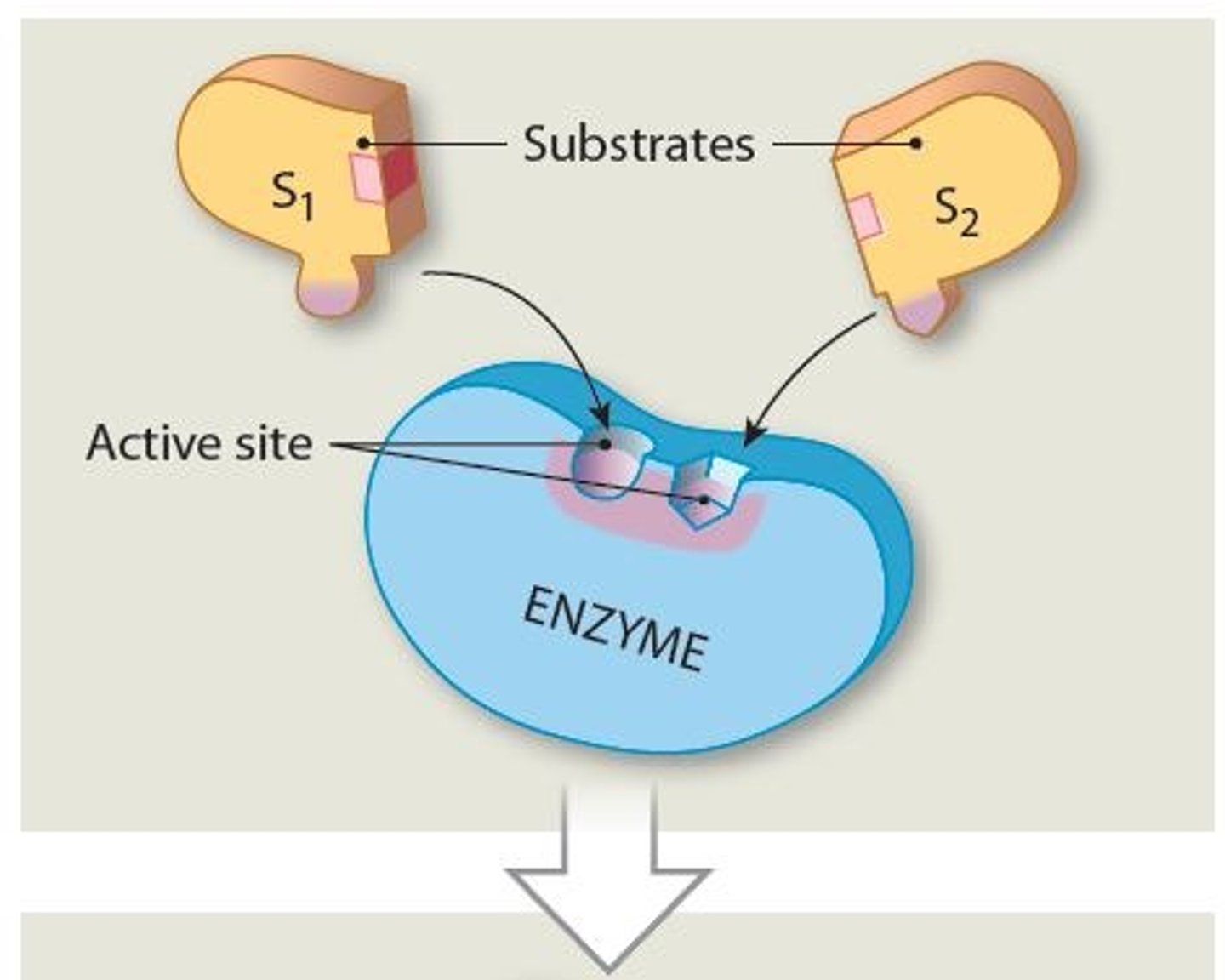
Where does substrate binding occur on the enzyme
The Activation site
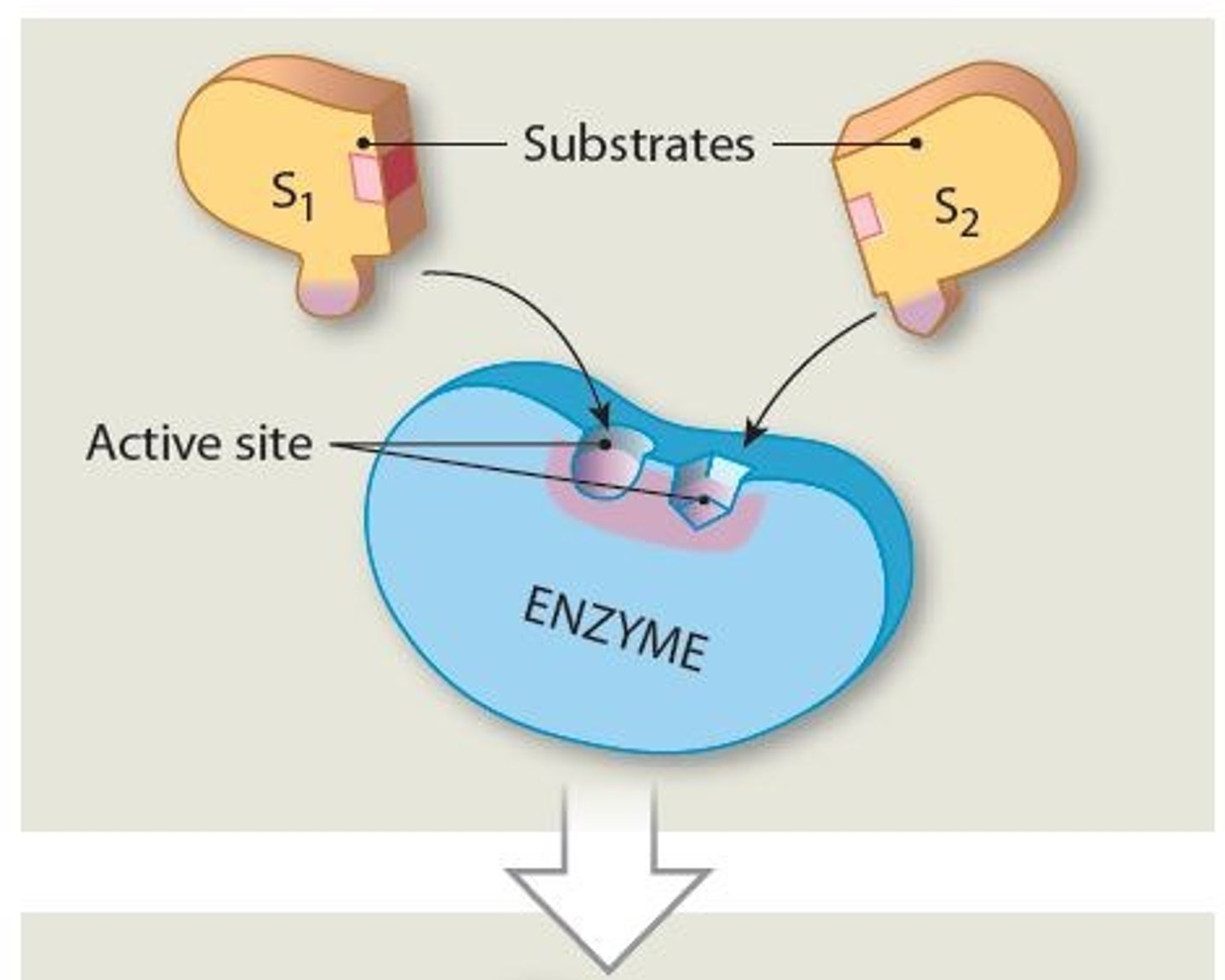
What are the reactants in an enzymatic reaction called?
Substrates
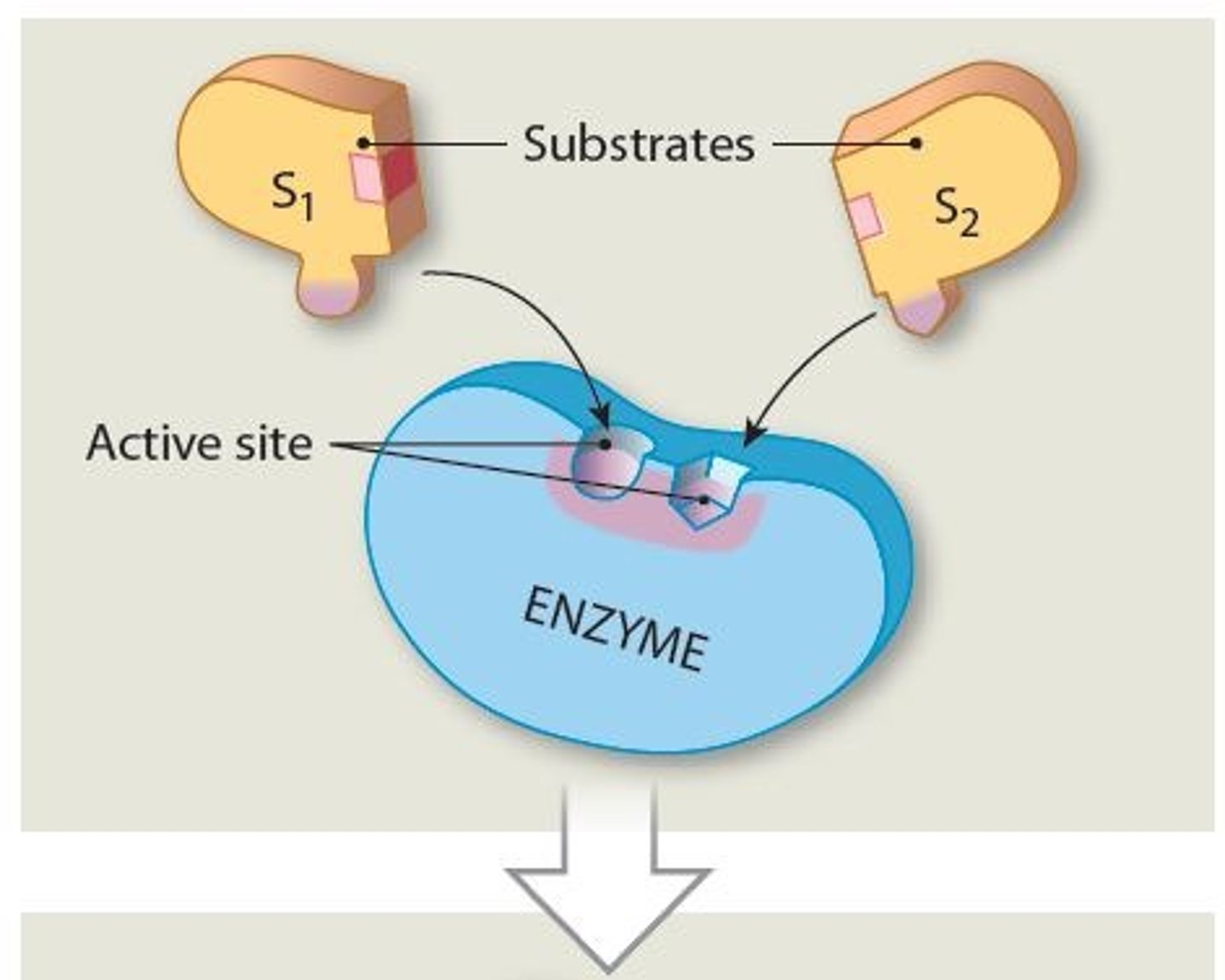
Active site
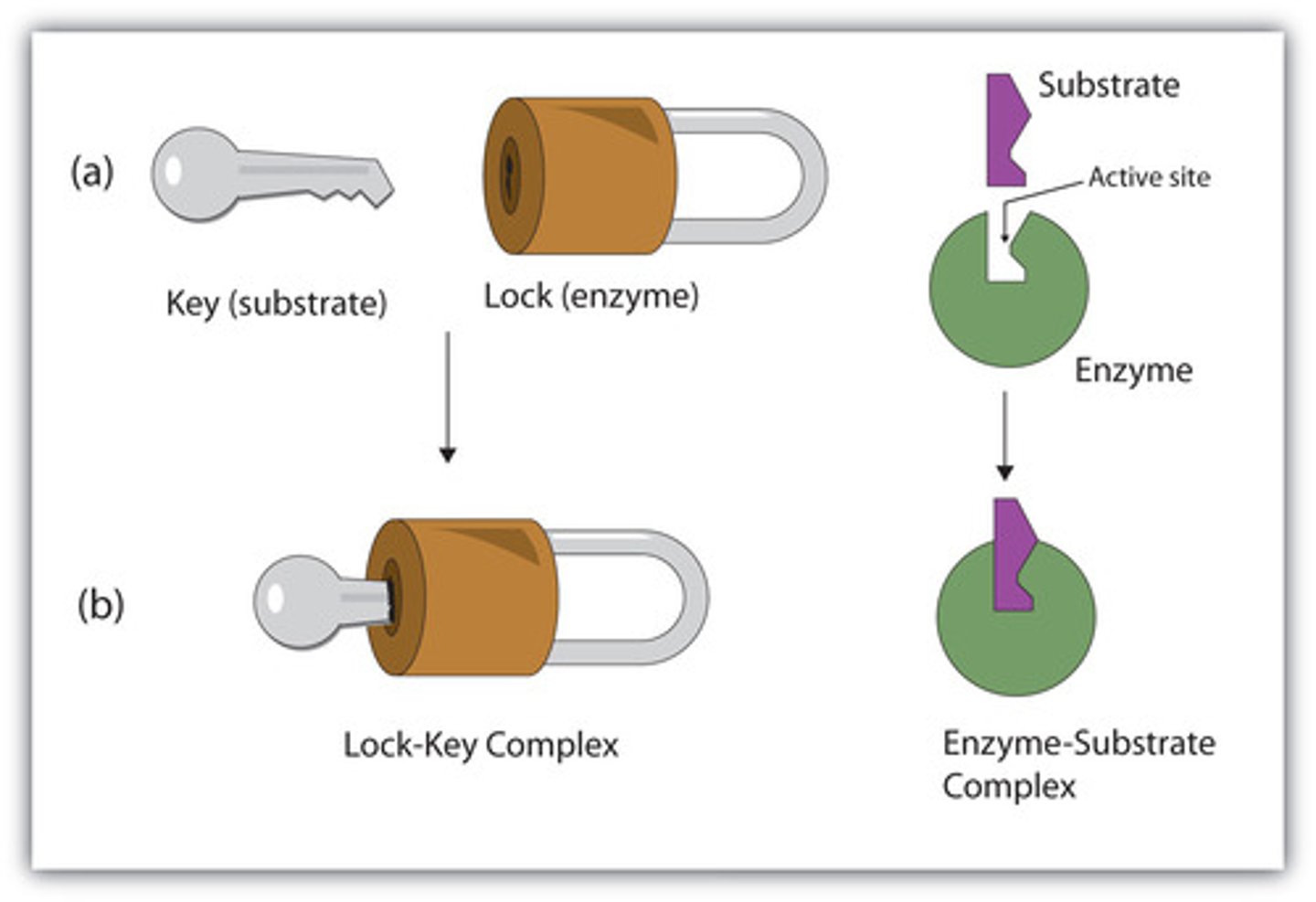
A specific region (groove or pocket) of an enzyme where substrates bind
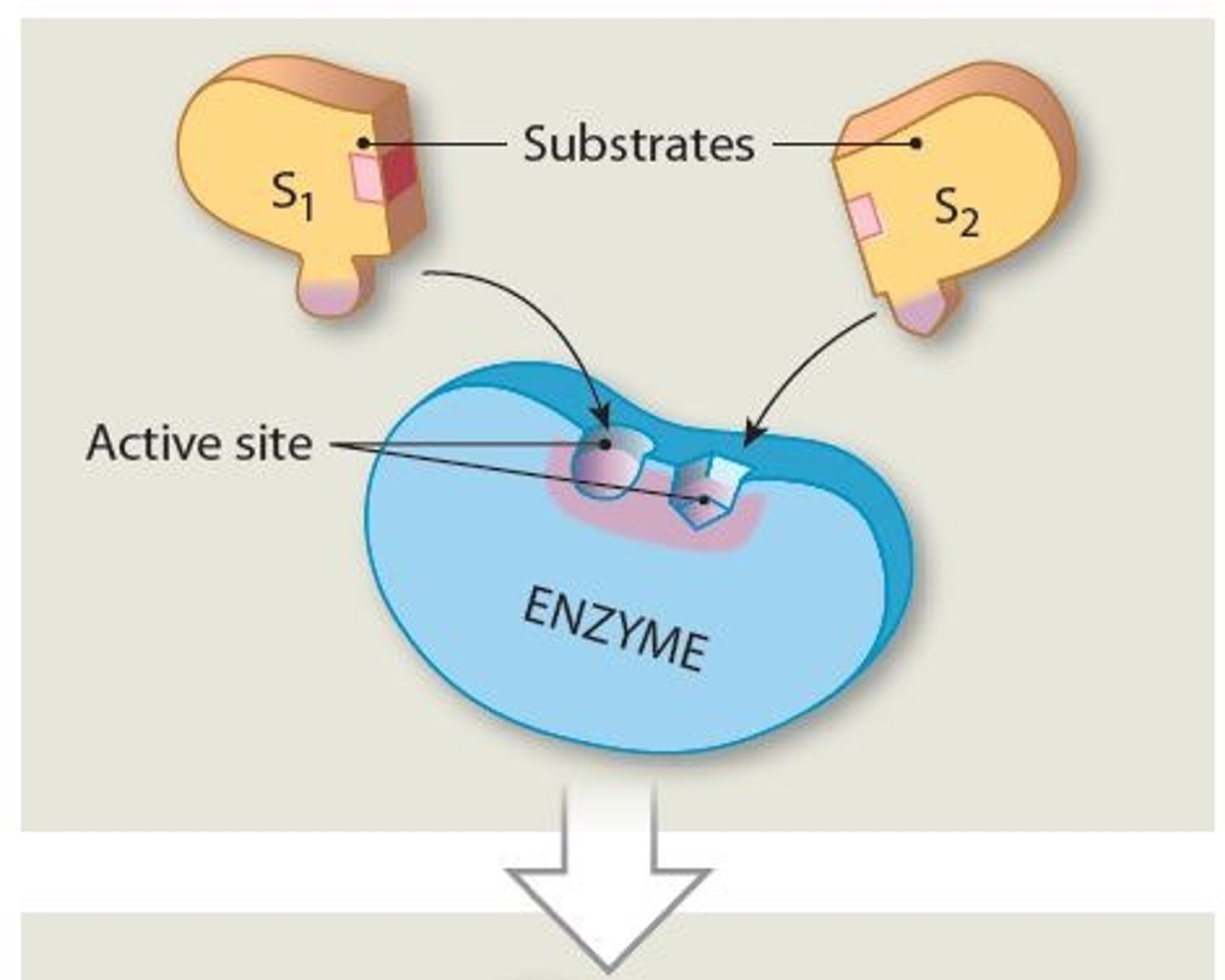
What determines the shape of the active site
The tertiary or quaternary structure of the enzyme molecule
Why must a Substrates bind to the active site of the enzyme
So an enzyme can function as a Catalyst (characteristic called Specificity), thus starting the enzymatic reactions
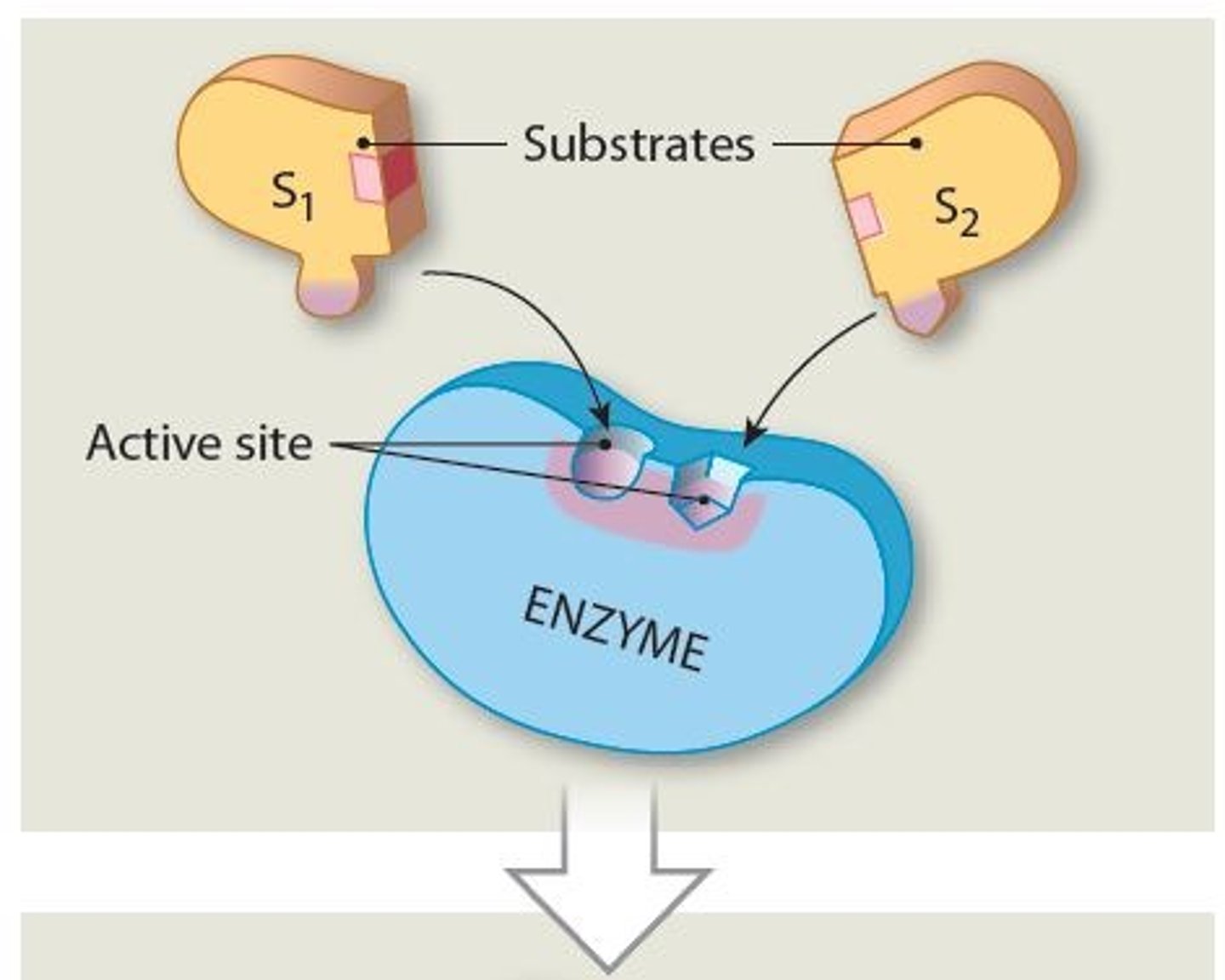
An enzyme's specificity is determined by?
The ability of its active sites to bind only to substrates with particular shapes and charges
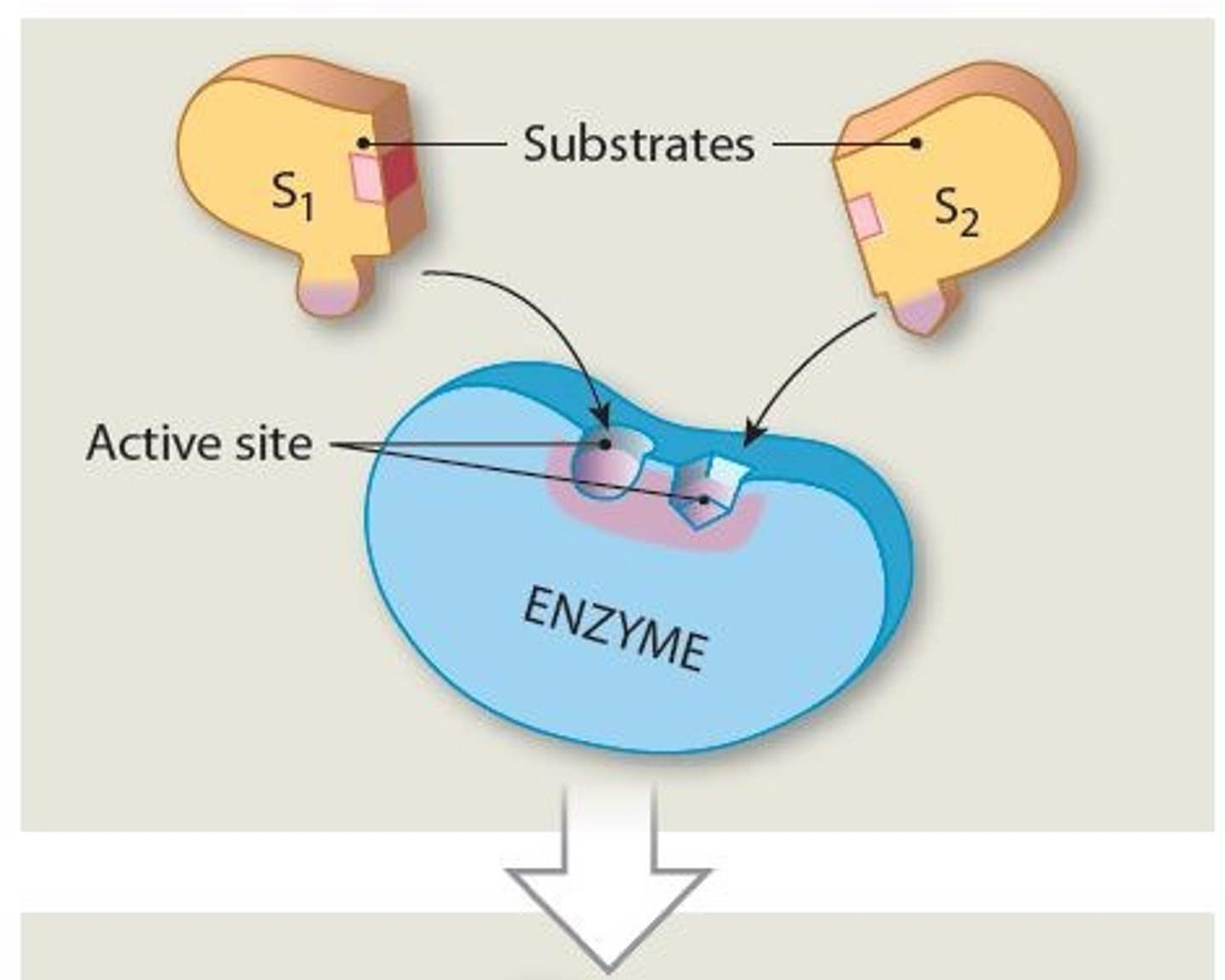
Enzymes overall process
1. Substrate binds to active site on enzyme, forming
enzyme-substrate complex
2. Substrate binding results in a temporary, reversible
change in shape of enzyme
3. Completed product detaches from the active site
4. Enzyme is able to repeat process
Saturation limits
The substrate concentration required for the enzyme to reach maximum rate of reaction (enzyme's maximum work rate)
Enzyme control of reaction rates
- Multiple enzymes in each cell
- Each enzyme is active under its own set of
conditions
- anything that changes the tertiary or quaternary shape of an enzyme can turn it "on" or "off" by changing the properties/shape of the active site and preventing formation of an enzyme substrate complex.
- Enzyme activation or inactivation is important
method of short-term control over reaction rates and
pathways
Relate an enzyme's structure to its reaction specificity.
An enzyme's specificity results from the unique shape of its active site, which permits only a substrate with a complementary shape to bind, producing an enzyme-substrate complex
High-energy compound
Donate energy to enzymatic reactions to form
products. _________________________ contains high-energy bonds, covalent bonds that release energy when broken
- Adenosine triphosphate (ATP) is the most common _____________________
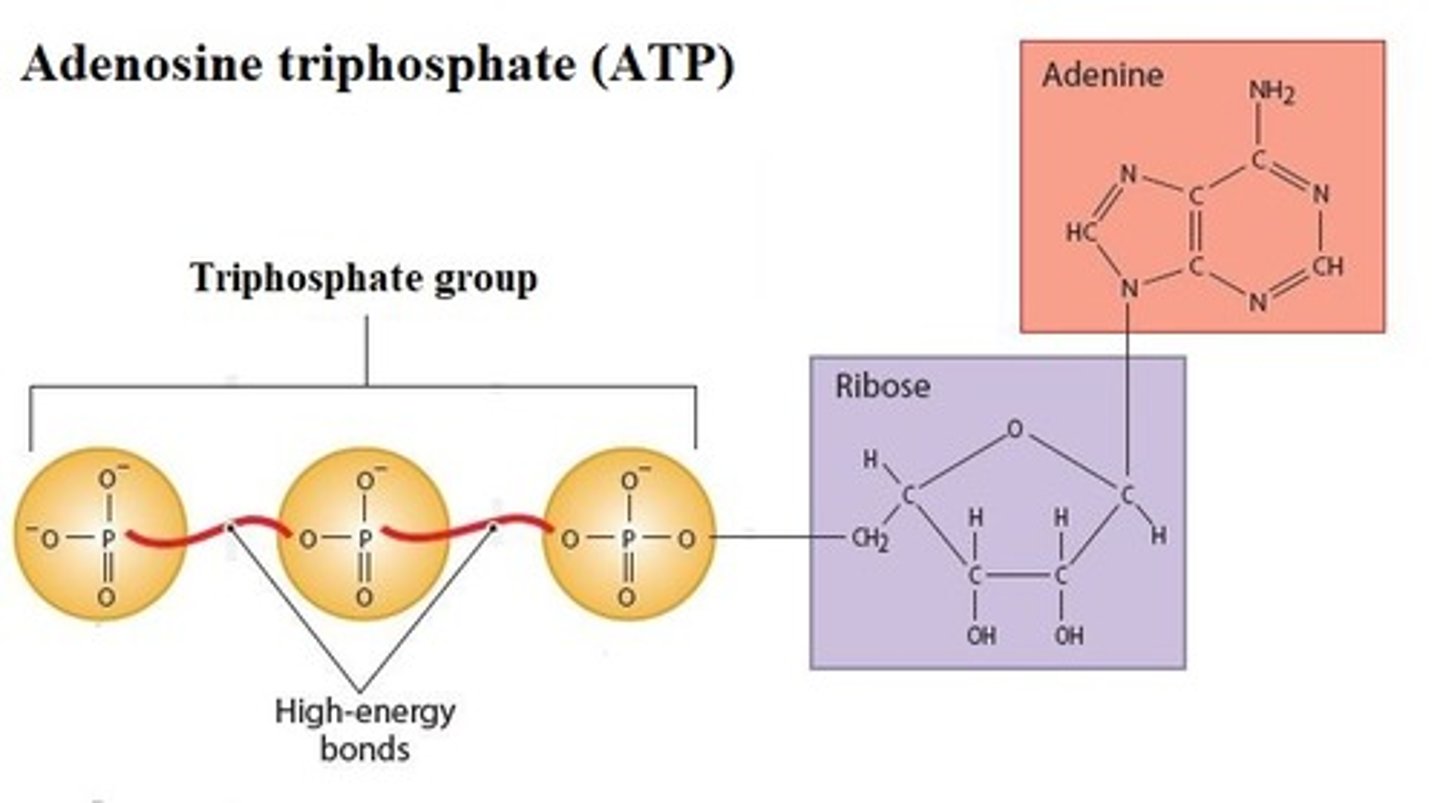
High-energy bonds
covalent bonds whose breakdown releases energy under controlled conditions
Most common high-energy compound
Adenosine triphosphate (ATP)
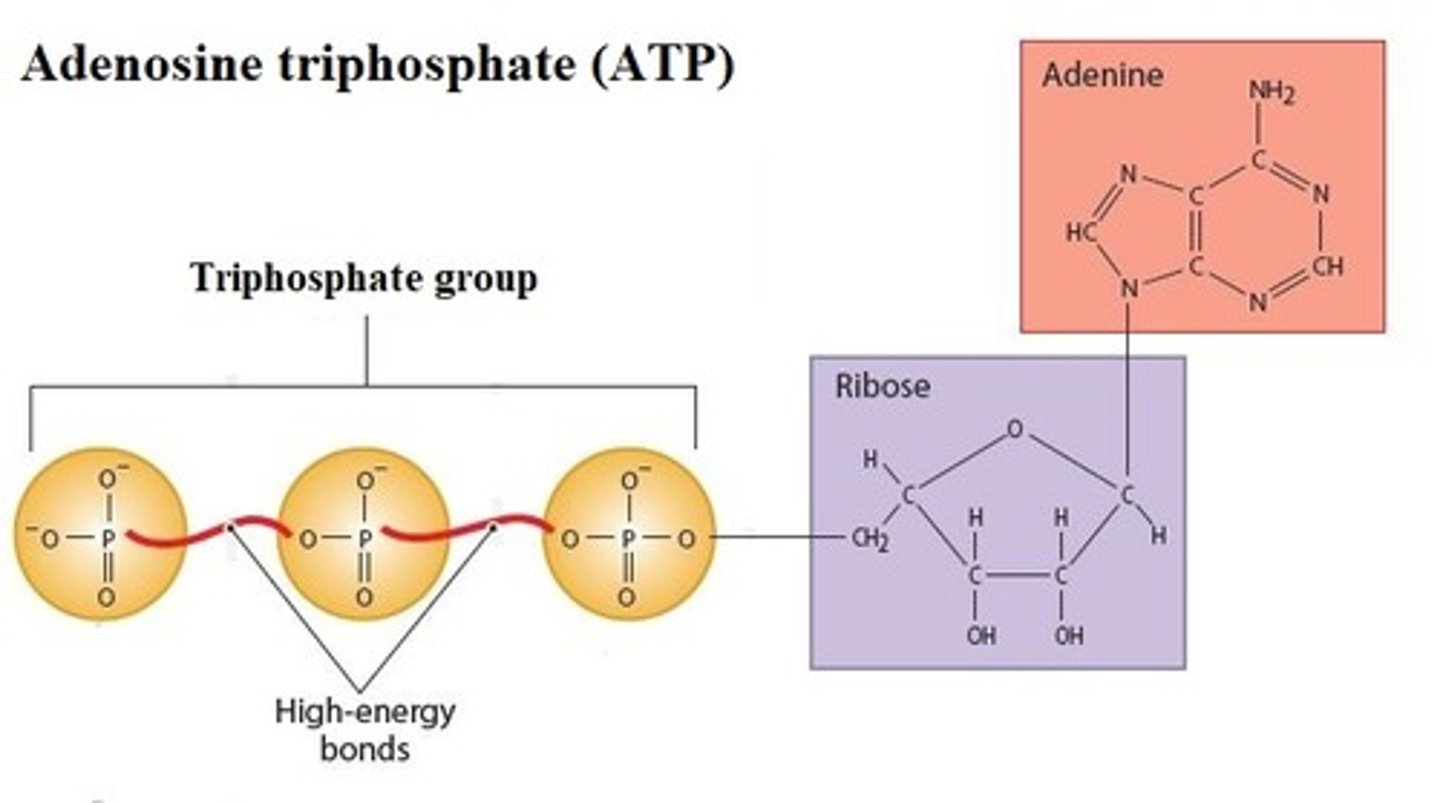
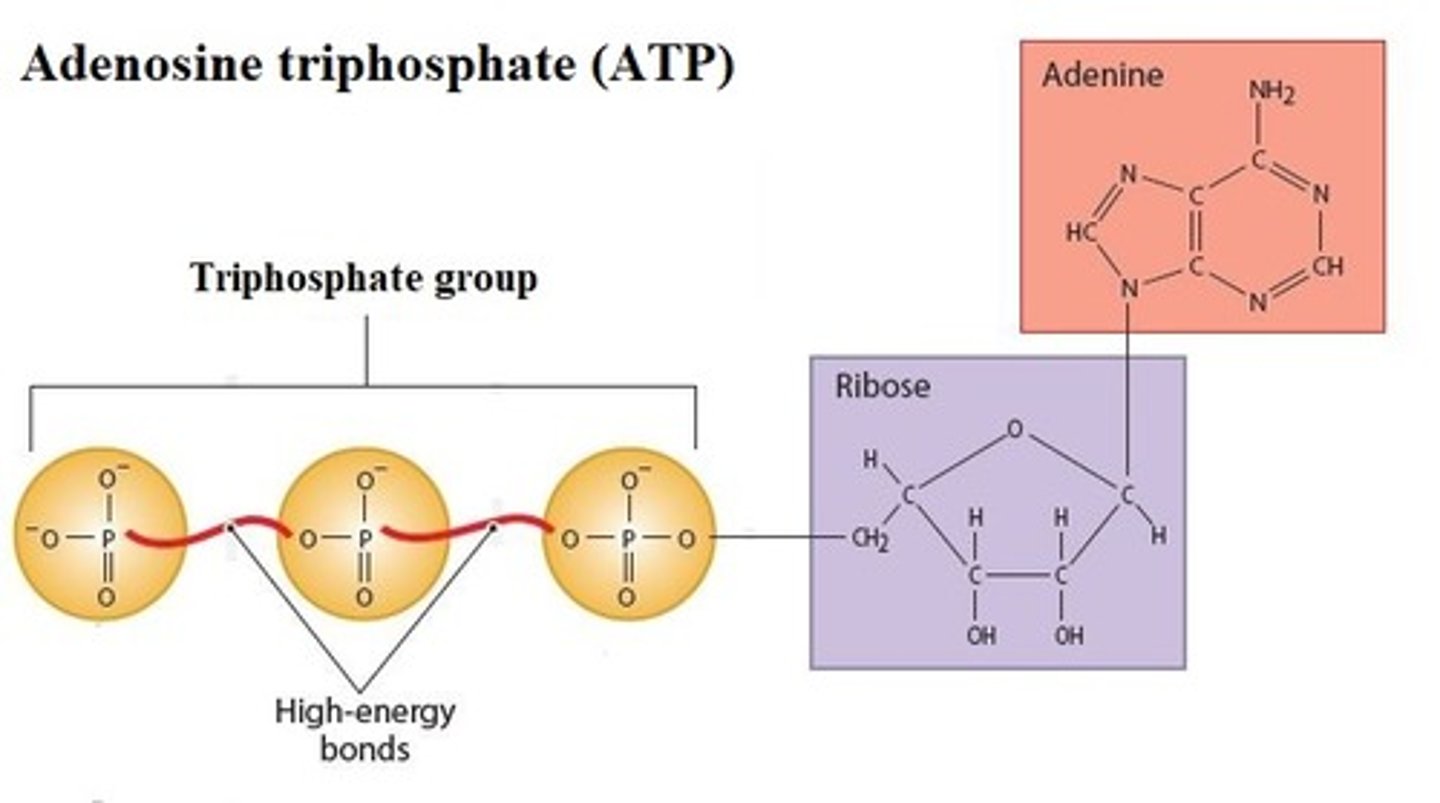
Describe Adenosine triphosphate (ATP)
A compound consisting of Adenosine linked to a Ribose and linked to a "Tri Phosphate" 3 phosphate groups; high-energy bond is required to attach the second and third phosphates
When product formation requires an energy donor, that donor is typically a
High-energy compound (ATP it the most common)
Where do cells obtain the energy needed for their vital functions?
From the high-energy bonds of compounds, such as Adenosine triphosphate (ATP)
ATP and ADP cycle
ATP (high energy) loses phosphate (energy released), creating ADP (low energy). Then, the ADP gains phosphate (energy gained), turning back into ATP and the cycle continues.
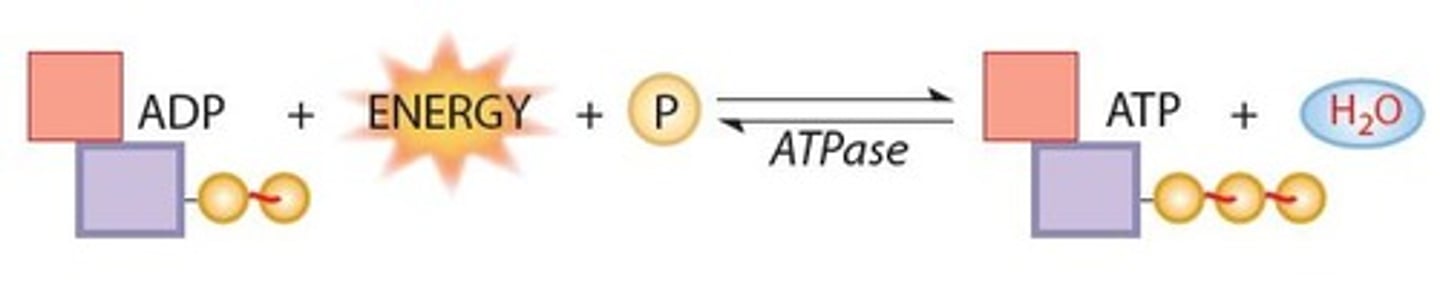
What are the products of ATP hydrolysis?
ADP + Energy + P
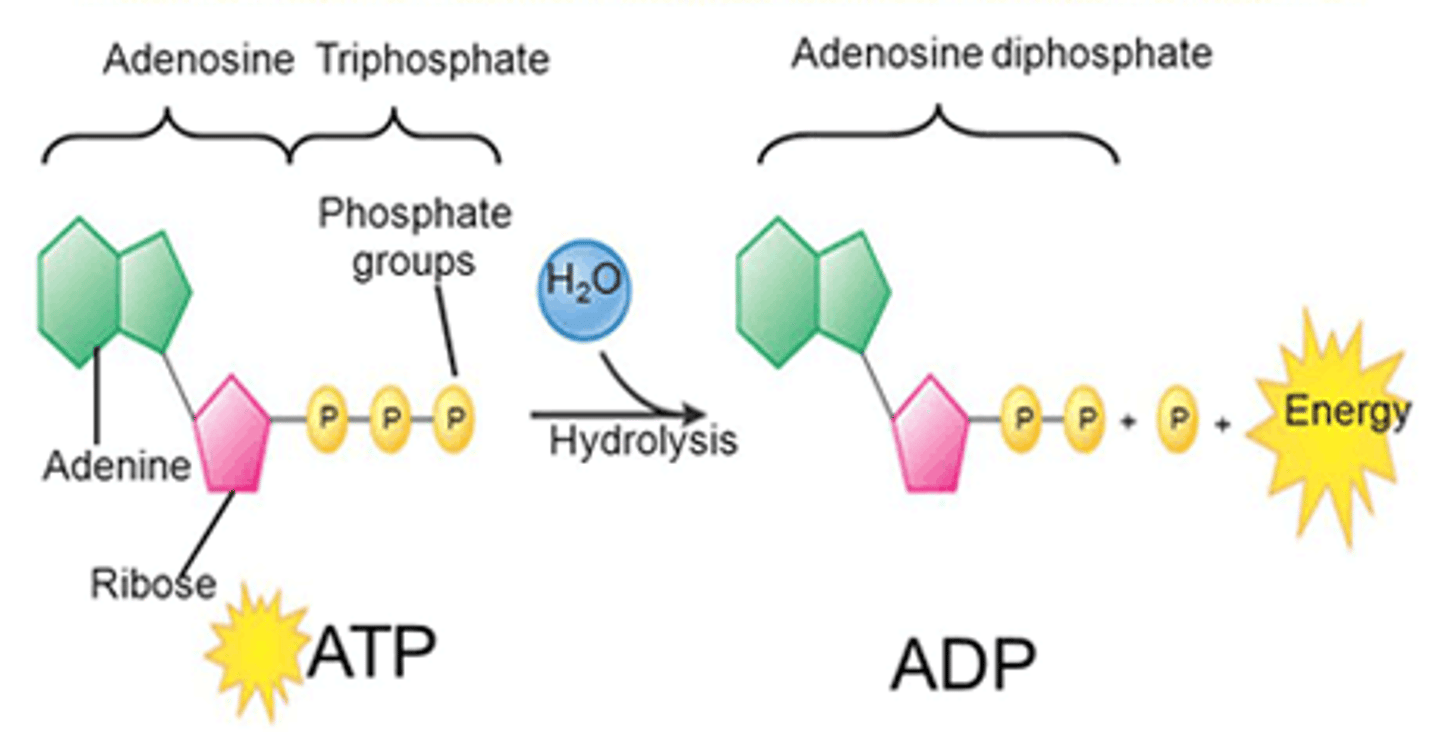
Adenosine Triphosphatase (ATPase)
The enzyme that catalyzes the breaking down of ATP to ADP + P + energy
How is energy obtained?
By breaking down organic substrates
Carbohydrates are an example of organic compounds whose chemical characteristics result from which functional group?
A. amino group
B. hydroxyl group
C. phosphate group
D. carboxylic acid group
B
Which organic compounds, containing a carbon-to-hydrogen ratio near 1:2, function as chemical messengers, coordinating local cellular activities?
A. carbohydrates
B. eicosanoids
C. fatty acids
D. proteins
B
The intertwining of three linear subunits in collagen to form a fibrous protein is an example of which structural level of proteins?
A. tertiary
B. secondary
C. quaternary
D. primary
C
Which statement regarding high-energy compounds is INCORRECT?
A. The energy stored when ATP forms is released when it breaks down to ADP.
B. Cells can synthesize ATP in one location and then break it down in another.
C. The formation of ATP from ADP is an irreversible reaction.
D. ATP and other high-energy compounds provide the energy for the contraction of muscles and many enzymatic reactions.
C
Which of the following lipids do cells need to maintain their plasma membranes?
A. cholesterol
B. prostaglandins
C. leukotrienes
D. eicosanoids
A
Alpha helices are an example of which level of protein structure?
A. secondary structure
B. primary structure
C. tertiary structure
D. quaternary structure
A
Before an enzyme can function as a ________________ , the substrates must bind to a specific region of the enzyme
catalyst
The formation of ATP begins with __________________, an organic molecule consisting of a small ring-shaped organic molecule and a simple sugar.
adenosine
Match the functional group to its corresponding characteristic or function: Amino group, -NH2
A. Releases H+ to become R-COO-
B. Can accept or release H+, depending on pH
C. May link molecules through dehydration synthesis
D. May link other molecules to form larger structures, and may store energy
B
Match the functional group to its corresponding characteristic or function: Carboxyl group, -COOH
A. Releases H+ to become R-COO-
B. Can accept or release H+, depending on pH
C. May link molecules through dehydration synthesis
D. May link other molecules to form larger structures, and may store energy
A
Match the functional group to its corresponding characteristic or function: Phosphate group, -PO4
A. Releases H+ to become R-COO-
B. Can accept or release H+, depending on pH
C. May link molecules through dehydration synthesis
D. May link other molecules to form larger structures, and may store energy
D
Match the functional group to its corresponding characteristic or function: Hydroxyl group, -OH
A. Releases H+ to become R-COO-
B. Can accept or release H+, depending on pH
C. May link molecules through dehydration synthesis
D. May link other molecules to form larger structures, and may store energy
C
Match the lipid type to its primary function(s): Steroids
A. Function as energy sources
B. Function as energy sources, energy storage, insulation, and physical protection
C. Function as chemical messengers coordinating local cellular activities
D. Function as structural components of cell membranes, hormones, and digestive secretions in bile
E. Function as structural components of cell membranes
D
Match the lipid type to its primary function(s): Fatty acids
A. Function as energy sources
B. Function as energy sources, energy storage, insulation, and physical protection
C. Function as chemical messengers coordinating local cellular activities
D. Function as structural components of cell membranes, hormones, and digestive secretions in bile
E. Function as structural components of cell membranes
A
Match the lipid type to its primary function(s): Eicosanoids
A. Function as energy sources
B. Function as energy sources, energy storage, insulation, and physical protection
C. Function as chemical messengers coordinating local cellular activities
D. Function as structural components of cell membranes, hormones, and digestive secretions in bile
E. Function as structural components of cell membranes
C
Match the lipid type to its primary function(s): Glycerides
A. Function as energy sources
B. Function as energy sources, energy storage, insulation, and physical protection
C. Function as chemical messengers coordinating local cellular activities
D. Function as structural components of cell membranes, hormones, and digestive secretions in bile
E. Function as structural components of cell membranes
B
Match the lipid type to its primary function(s): Glycolipids
A. Function as energy sources
B. Function as energy sources, energy storage, insulation, and physical protection
C. Function as chemical messengers coordinating local cellular activities
D. Function as structural components of cell membranes, hormones, and digestive secretions in bile
E. Function as structural components of cell membranes
E
Match the protein level of structural complexity to its description:
Primary structure
A. The sequence of amino acids bonded together in a linear chain
B. Results from bonds between atoms at different parts of the polypeptide chain
C. Results primarily from interactions between the polypeptide chain and the surrounding water molecules
D. Results from the interaction between individual polypeptide chains to form a protein complex
A