Orgo 2 - up to test 1
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
Toluene
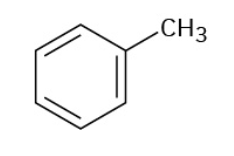
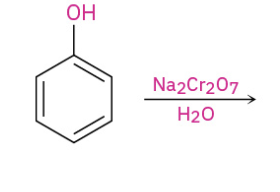
Phenol
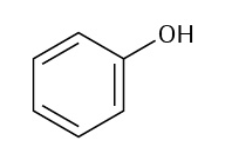
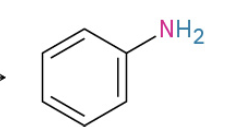
Aniline
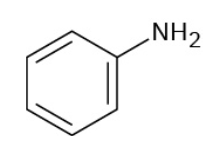
Acetophenone
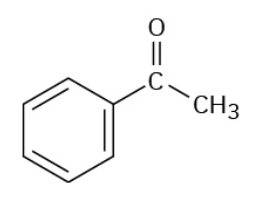
Benzaldehyde
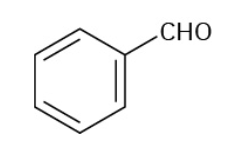
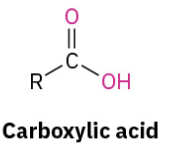
Benzoic Acid
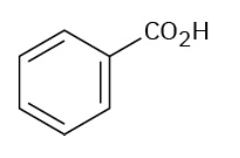
ortho-Xylene
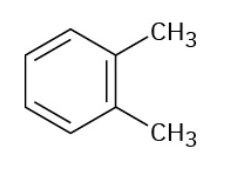
Stryrene
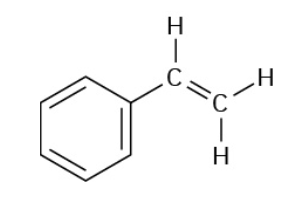
Fluorination
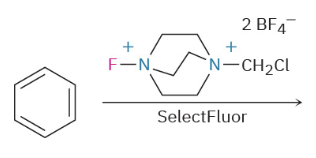
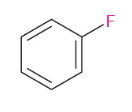
Bromination
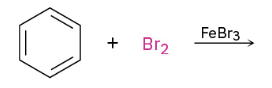
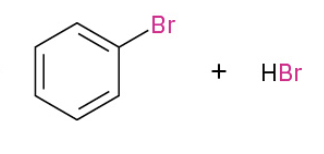
Chlorination
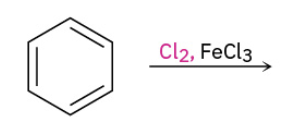
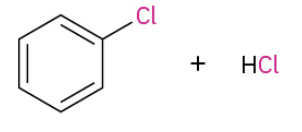
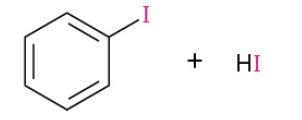
Iodination
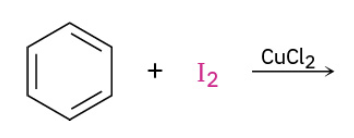
Nitration
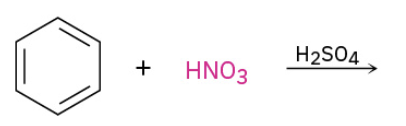
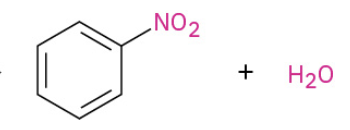
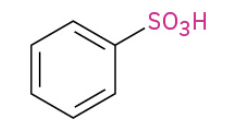
Sulfonation

Friedel-Crafts Alkylation
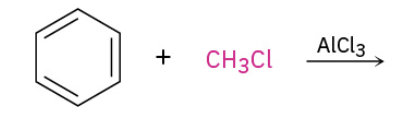
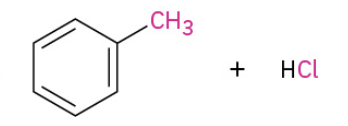
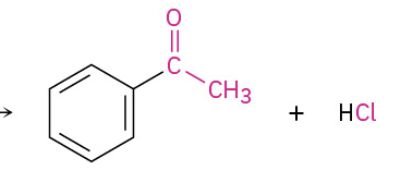
Friedel Crafts Acylation
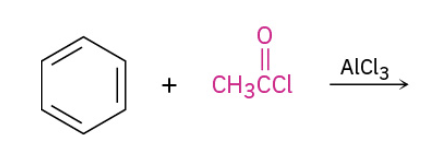
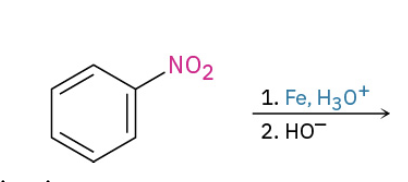
Reduction of aromatic nitro group
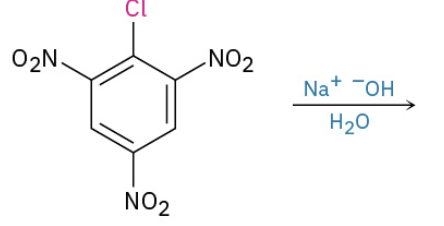
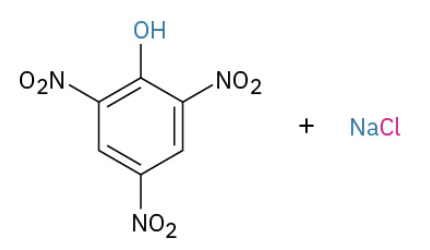
Nucleophilic Aromatic substitution by addition to activated halides
Works with HSO3 group as well instead of cl
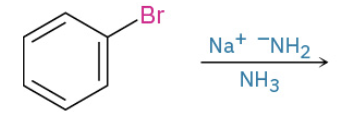
Nuc Aro Sub by form of benzene intermed
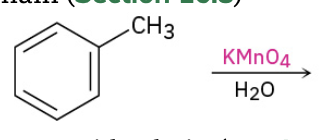
Oxidation of alkylbenzene side chain
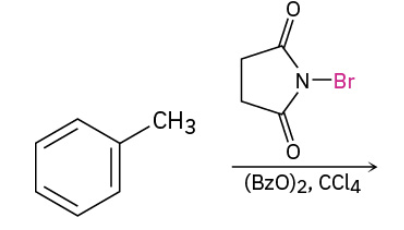
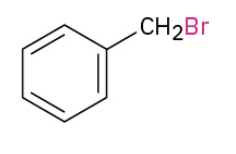
Benzylic bromination of alkylbenzene
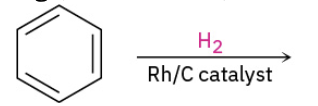
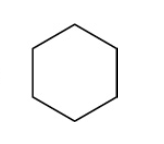
Catalytic hydrogenation of aromatic ring
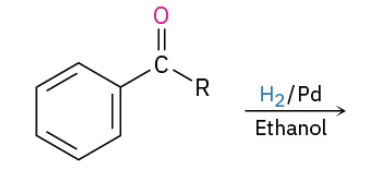
Reduction of aryl alkyl ketones
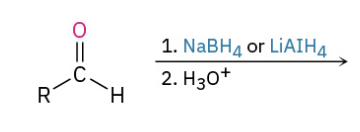
Reduction aldehyde
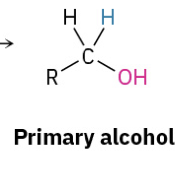
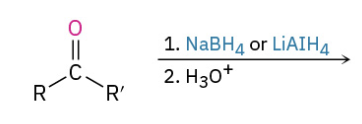
Reduction Ketone
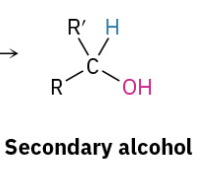
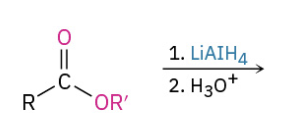
Reduction Ester
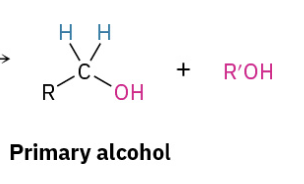
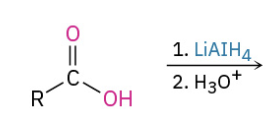
Reduction Carbox Acid
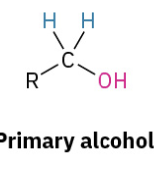
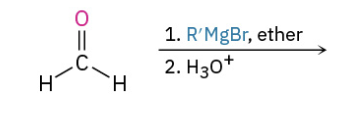
Grinyard Fromeldehyde
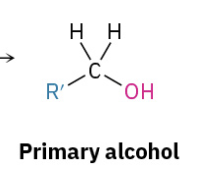
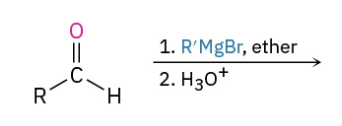
Grinyard Aldehyde
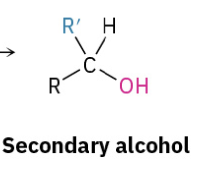
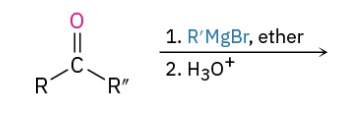
Grinyard Ketone
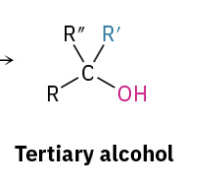
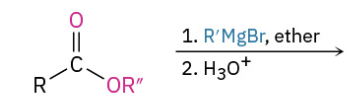
Grinyard Ester
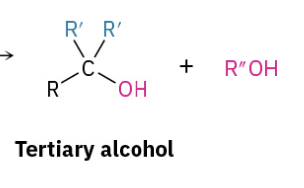
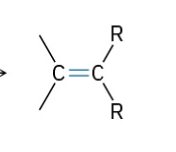
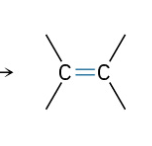
Dehydration
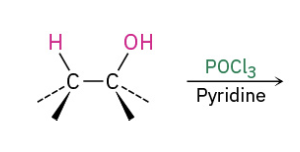
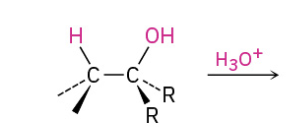
Dehydratin secondary and tertiary
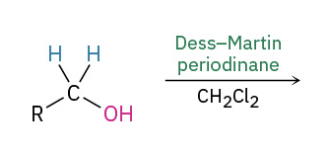
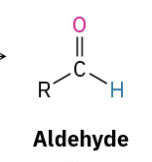
Oxidation primary alc
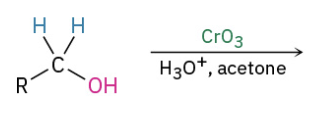
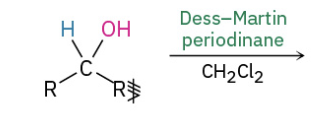
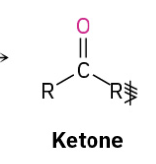
Secondary alc
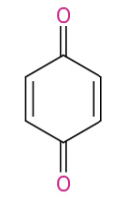
Carbox for primary
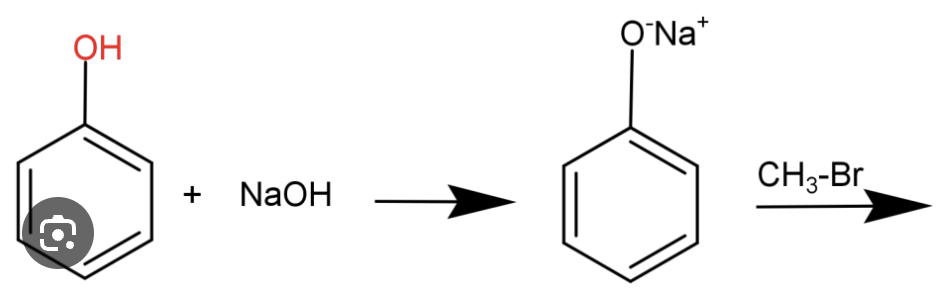
Phenol to anisole (to protect oh for friedel crafts)
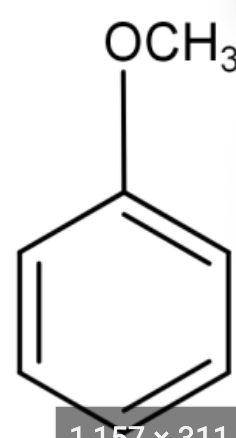
Deprotection of Anisole (Benzene - o - CH3) to form Phenol
BBr3 or HBr/Heat
Make Phenol from Aniline
Aniline starter
NaNO2, HCl in H2O
H2O + heat
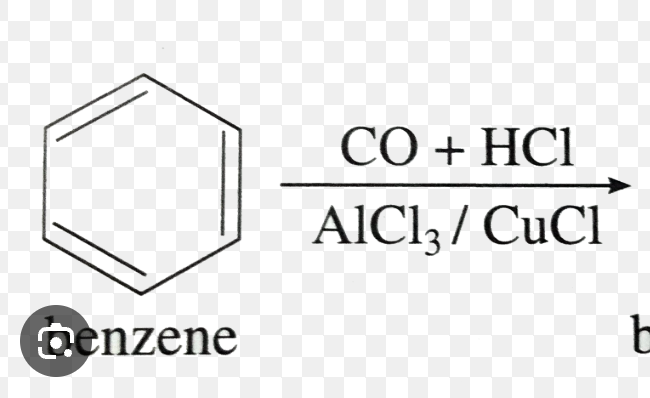
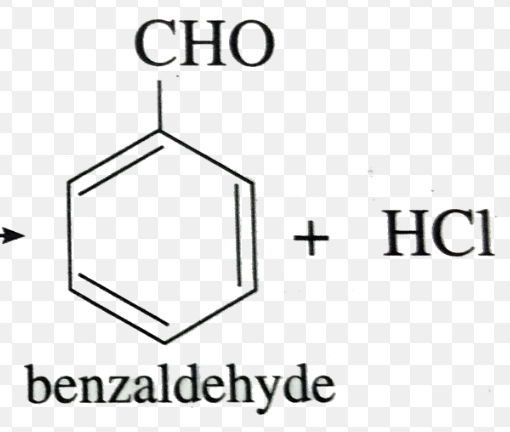
NH2 —> ClCOCH3 + Pyridine
H3O+, H2O, Heat
Stabilize NH2
Remove protecting group
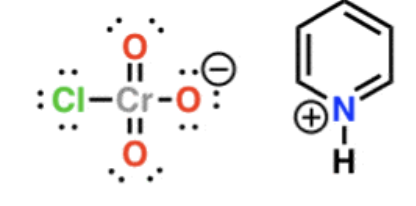
PCC (mild reducing agent)
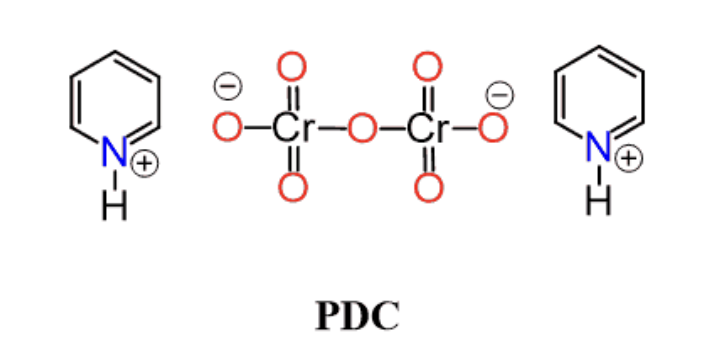
PDC (mild ox agent)
Protect OH then deprotect
TMS in Et2N
Remove with acid (3M with heat) in water
Remove HSO3
Dilute H2SO4 and heat