AP Chemistry Exam
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
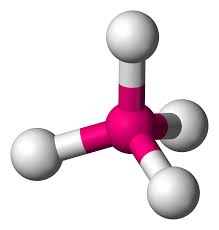
Tetrahedral
Atoms Touching:4
Lone Pairs:0
Bond Angle: 109.5
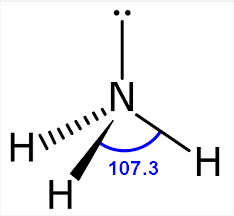
Trigonal Pyramidal
Atoms Touching: 3
Lone Pairs: 1
Bond Angle:~109.5
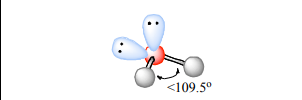
Bent
Atoms Touching: 2
Lone Pairs: 2
Bond Angle:~109.5
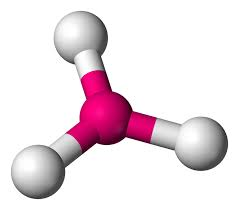
Trigonal Planar
Atoms Touching: 3
Lone Pairs: 0
Bond Angle: 120
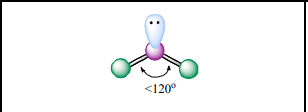
Bent / Angular
Atoms Touching: 2
Lone Pairs: 1
Bond Angle:~120
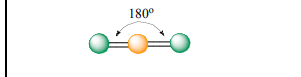
Linear
Atoms Touching: 2
Lone Pairs: 0
Bond Angle: 180
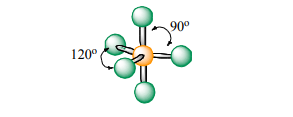
Trigonal Bipyramidal
Atoms Touching: 5
Lone Pairs: 0
Bond Angle: 90 & 120
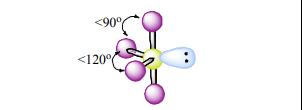
See-Saw
Atoms Touching: 4
Lone Pairs: 1
Bond Angle:~90 & 120
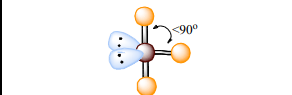
T-Shaped
Atoms Touching: 3
Lone Pairs: 2
Bond Angle: <90
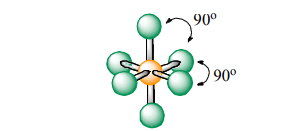
Octahederal
Atoms Touching: 6
Lone Pairs: 0
Bond Angle: 90
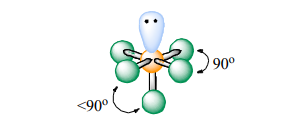
Square Pyramidal
Atoms Touching: 5
Lone Pairs: 1
Bond Angle:~90
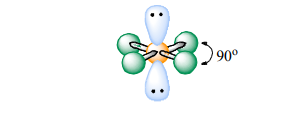
Square Planar
Atoms Touching: 4
Lone Pairs: 2
Bond Angle: 90
Hydrogen Bonding
Molecules containing O-H, N-H, or F-H bonds
Ionic Bonding
Occurs between a metal and a nonmetal when electrons are transferred, forming ions that attract each other
Covalent Bonding
Involves the sharing of electrons between nonmetals
Metallic Bonding
Characterizes metals, where valence electrons are delocalized and shared among all atoms
Polar Covalent Bonds
Form when electrons are shared unequally due to differences in electronegativity, resulting in partial charges on the bonded atoms
London Dispersion Forces
Weak, temporary attractions between all molecules due to temporary dipoles
Microwave Radiation
Causes Rotation of Molecules
Infrared Radiation
Causes Vibration of Molecules
UV / Visible Radiation
Causes electrons to transition to different energy levels
Solubility Rules
S- Sodium Na+
N- Nitrate NO3-
A - Ammonium NH4+
P- Potassium K+
Bond Enthalpies
H = Bonds Broken - Bonds Formed
(left - right side)
Strong Acids
HCl
HBr
HI
HNO3
H2SO4
HClO4
Strong Bases
Group 1 and 2 Hydroxides
LiOH
NaOH
KOH
Ca(OH)2
Sr(OH)2
Ba(OH)2
Dilution Equation
M1V1 = M2V2
Titration Equation
MaVa=MbVb
Titration Curves
@ Half-equivalence point, pH=pKa
Percent Error
|Calculated Answer - Correct Answer|/correct answer x 100
H<0
exothermic
H>0
endothermic
G<0
TDF
G>0
Non TDF
Thermodynamically Favored
H S
– + All temperatures
+ – Never
+ + At higher temperatures
– – At lower temperatures
Red Cat and An OX
Reduction takes place at cathode
Oxidation takes place at the anode
OIL RIG
Oxidation is Losing
Reduction is Gaining
Salt Bridge
Anions to Anode, Cations to Cathode