Cracking
1/22
Earn XP
Description and Tags
-some relavent bits from videos- comprehensive/important info there -summaries from each vid -one catch out qu/unuslanon strait forward qu from ques sections
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
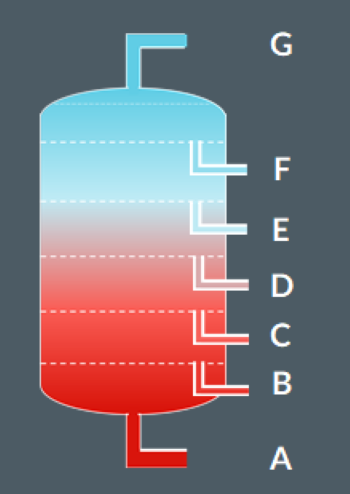
During fractional distillation, oil is separated into…
A: Bitumen
B: Fuel Oil
C: Lubricating Oil
D: Diesel Oil
E: Kerosene/Parfin
F: Petrol/Gasoline
G: Gases
As we go down the fractionating column, we get hydrocarbons with carbon chains that are…
longer
Why are shorter chain carbons more useful….
more flammable/burn easier
high energy output when burnt eg) burning a litre of petrol produces more energy than of bitumen.
Cracking is the process of…
taking a sample from one of the big oil fractions/longer chain hydrocarbons and breaking them into smaller-chain hydrocarbons.
Unlike most other reactions, cracking does not involve specific reactant creating specific products
Thermal cracking exposes long-chain alkanes to…….temperatures of ……(°C) and…… (atm/kPa)pressures.
high
700→ 1000 ºC
high
70 atm / 7000 kPa
When long-chain hydrocarbons are thermally cracked, they produce one …….. and one or more……
alkane
alkenes
Alkanes produced in thermal cracking are mainly…
linear
In addition, the …… produced in thermal cracking can be used to produce…
alkenes
platics
When we write a full equation for a thermal cracking reaction, we first write an……….. with the information that we’re given.
Second, we work out the of………. the hydrocarbon that is missing.
Third, we calculate the……… between the atoms in the products and the ……...
Fourth, we write out the full……… for the thermal cracking reaction.
equation
type
difference
reactants
equation
At high temperatures, an unknown compound is converted into one molecule of ,C5H10, one molecule of C6H14 and one molecule of C8H16.
?→C5H10 + C6H14 +C8H16.
C=19 H=40
C19H14→ C5H10 + C6H14 +C8H16.
IUPAC name of Reactant: Nonane
The process of breaking bonds is …….
Thats why we say thermal cracking requires lots of …..
As …… is needed from the……..
endothermic
heat energy
energy
surroundings (to break bonds)
sometmes thermal cracking can produce……
branched alkanes
Catalytic cracking uses a ……catalyst.
It requires ….temperatures of…… degrees celsius and ……pressures of …..atm/……..kPa.
zeolite
high
525-700
low
1 atm/ 100 kPa
Why is the zeolite catalyst hetrogenous
the hydrocarbon is in the liquid phase (aq) and the catalyst is in the solid phase (s)
therefore its bcs its in a different state than the reactants
what do catalyst do
increase rate of reaction by providing an alternate route/ lowering Ea
What is zeolite made of
aluminum + silicone
‘aluminosillicate’
What are the products of catalytic cracking
one or more cycloalkanes
mainly branched alkanes
aromatic compounds eg) benzene C6H6
When we write a full equation for a catalytic cracking reaction with an unknown product, we first write…….. an with the information that we’re given.
Second, we work out the…… of the hydrocarbon that is missing.
Third, we calculate the…… between the atoms in the products and the ……..
Fourth, we write out the full …….for the catalytic cracking reaction.
equation
type eg) branched alkane or cycloalkane
difference
reactants
molecular formula equation
At high temperatures, C14H30 is catalytically cracked to form one molecule of cyclopropane, one other cycloalkane with 6 carbons and one alkane.
Give the IUPAC name of this alkane if it’s linear.
Give the IUPAC name of this alkane if it’s branched at the second carbon.
the missing product is a ‘branched’ alkane (given theres 2 cycloalkane) → C=5 H=12
Pentane
2-methylbutane
catalytic cracking can’t never produce …
alkenes
The benefit of thermal cracking is that it also produces…
Which can be used in …
and mainly …… which are …. chained
BUT (disadvantage) requires …… & ……
menaing it can be …..
alkenes
plastics
more valuble alkenes
shorter
high temperature
high pressure
costly (requires a lot of energy)
The benefit of catalytic cracking is that it uses …… which …. the rate of reaction and ….. by ‘……’
which allow the cracking to be …… & …….
requiring … energy due to …. temepratures and pressure in contast with thermal cracking
its main byproduct is ……. ……… which can be used as …..
BUT (disadvantage) requires …… to be constantly …..
menaing it can be …..
zeolite
lowers
Ea
providing an alternate route
cheaper
easier
less
lower
branched alkanes
fuels
zeolite catalyst
replaced
costly
Other than cracking, give one common use of alkane X.
Jet fuel or diesel or diesel oil or (motor) fuel or lubricant or lubricating oil or petrochemicals or kerosene or paraffin or central heating fuel or fuel oil