BJU Physical Science (6th ed) - Chapter 7
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
42 Terms
chemical reaction
a process where two or more substances are changed into one or more new substances
chemical equation
a combination of chemical formulas and symbols that models a chemical reaction
reactants
the substances that enter into a chemical reaction
products
the substances that the reactants combine to produce
coefficient
the number in front of an element symbol
subscript
the small lowercase number after an element symbol
synthesis/combination
a reaction that combines two or more reactants to yield a single product
decomposition
a reaction that is the opposite of the synthesis reaction that takes a single reactant and breaks it down into two or more products
combustion
a reaction that involves a fuel and oxygen as the reactants and yields CO2 and H2O
double replacement
a reaction where both the cations and anions swap places
single replacement
a reaction where one element in a compound is replaced by another element
oxidation
when a substance loses electrons in a chemical reaction
reduction
when a substance gains electrons in a chemical reaction
redox
reactions in which elements are oxidized and reduced are called
releases energy
exothermic reactions _______________________________________ as heat
absorb
endothermic reactions _______________________ energy
activation energy
the minimum energy needed to get a chemical reaction started
reaction rate
the speed of a reaction
catalyst
a substance that helps a reaction happen faster by lowering the activation energy
inhibitor
a substance that slows down a reaction by reducing the effectiveness or blocking the catalyst
reversible reactions
reactions that go forward and backward to maintain or restore equilibrium
equilibrium
the state when a system is balanced and influences cancel one another out
bubbles, precipitate, energy released, color change, odor, temperature change, burning, composition change
evidences of a chemical reaction
yields
The arrow symbol in a chemical equation, like the equal sign in math, means _______________.
Law of Conservation of Matter
What law states the matter cannot be created or destroyed?
Law of Conservation of Energy
What law states the energy cannot be created or destroyed, but can only be transferred or transformed.?
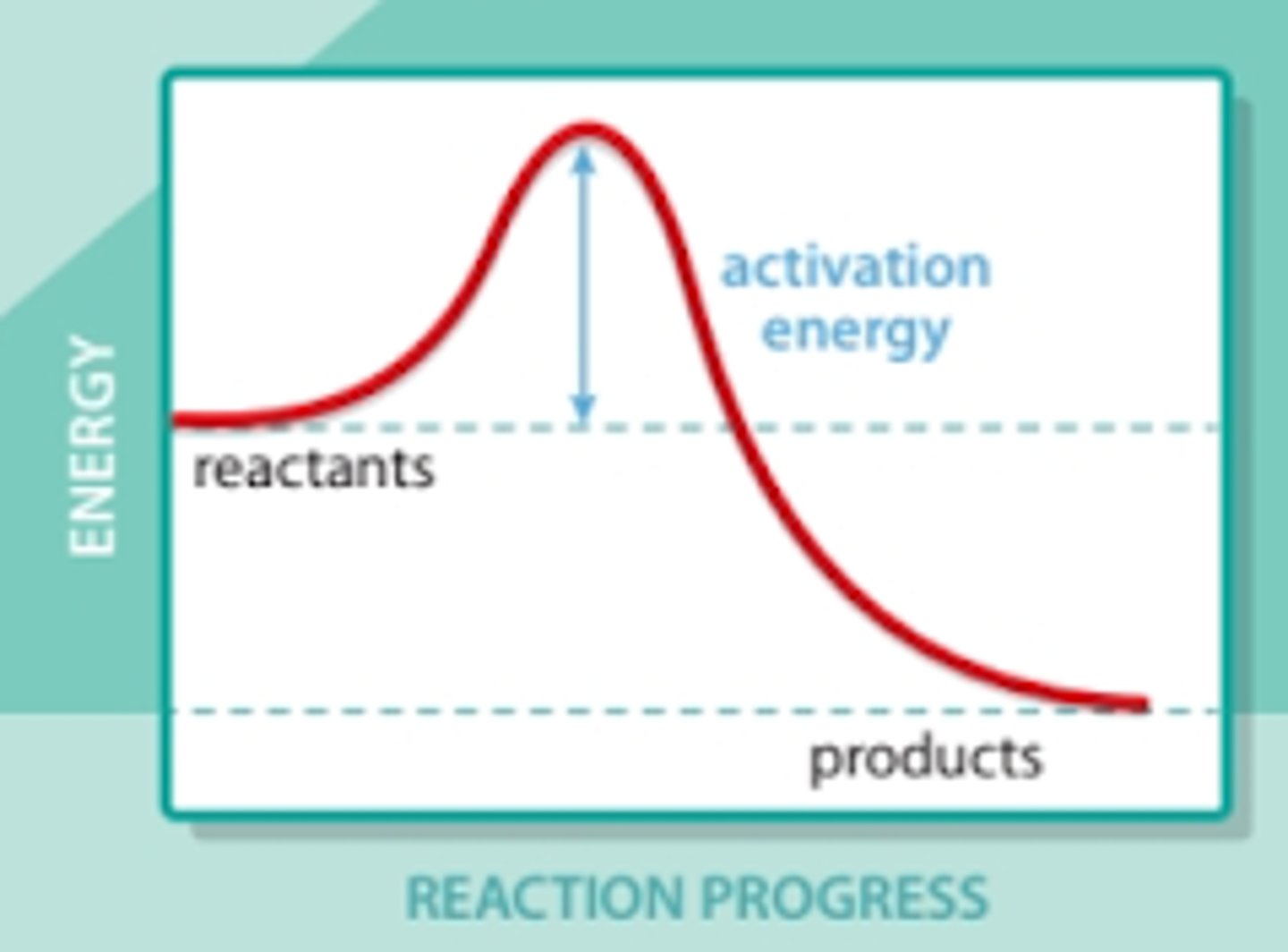
exothermic reaction
What type of reaction is this?
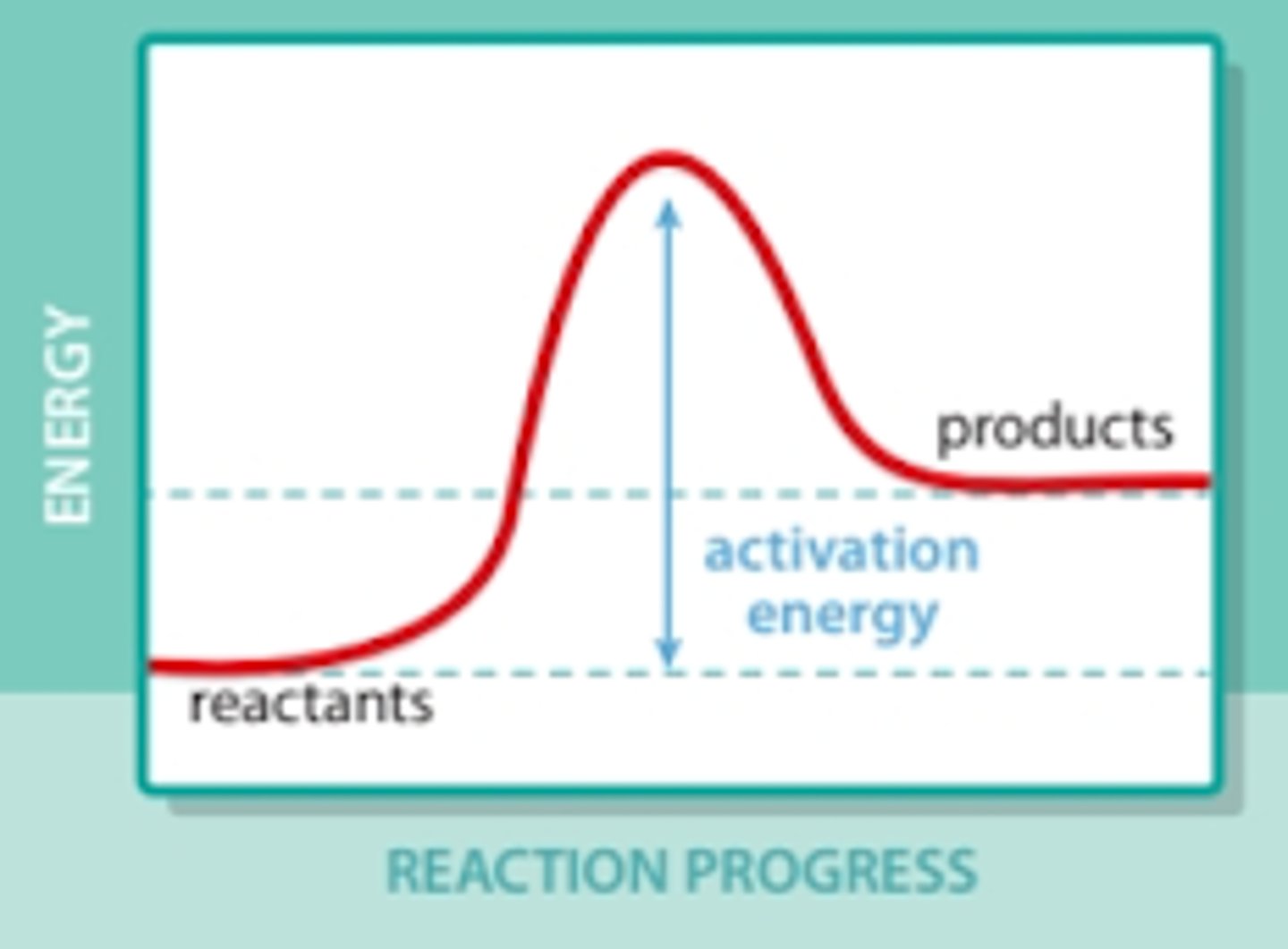
endothermic reaction
What type of reaction is this?
endothermic
Photosynthesis is an example of what type of reaction?
exothermic
Combustion is an example of what type of reaction?
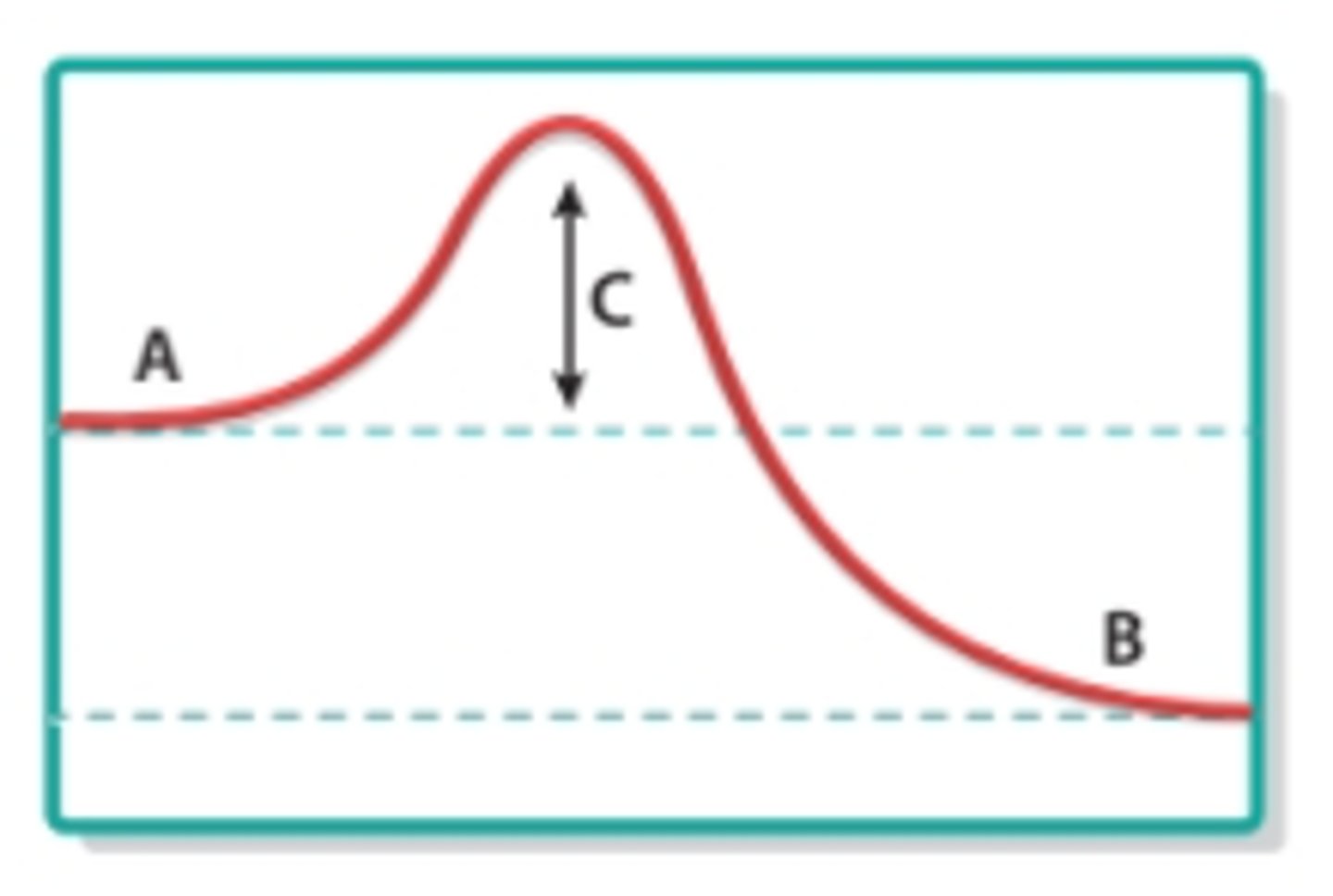
reactants
What does A represent in this image?
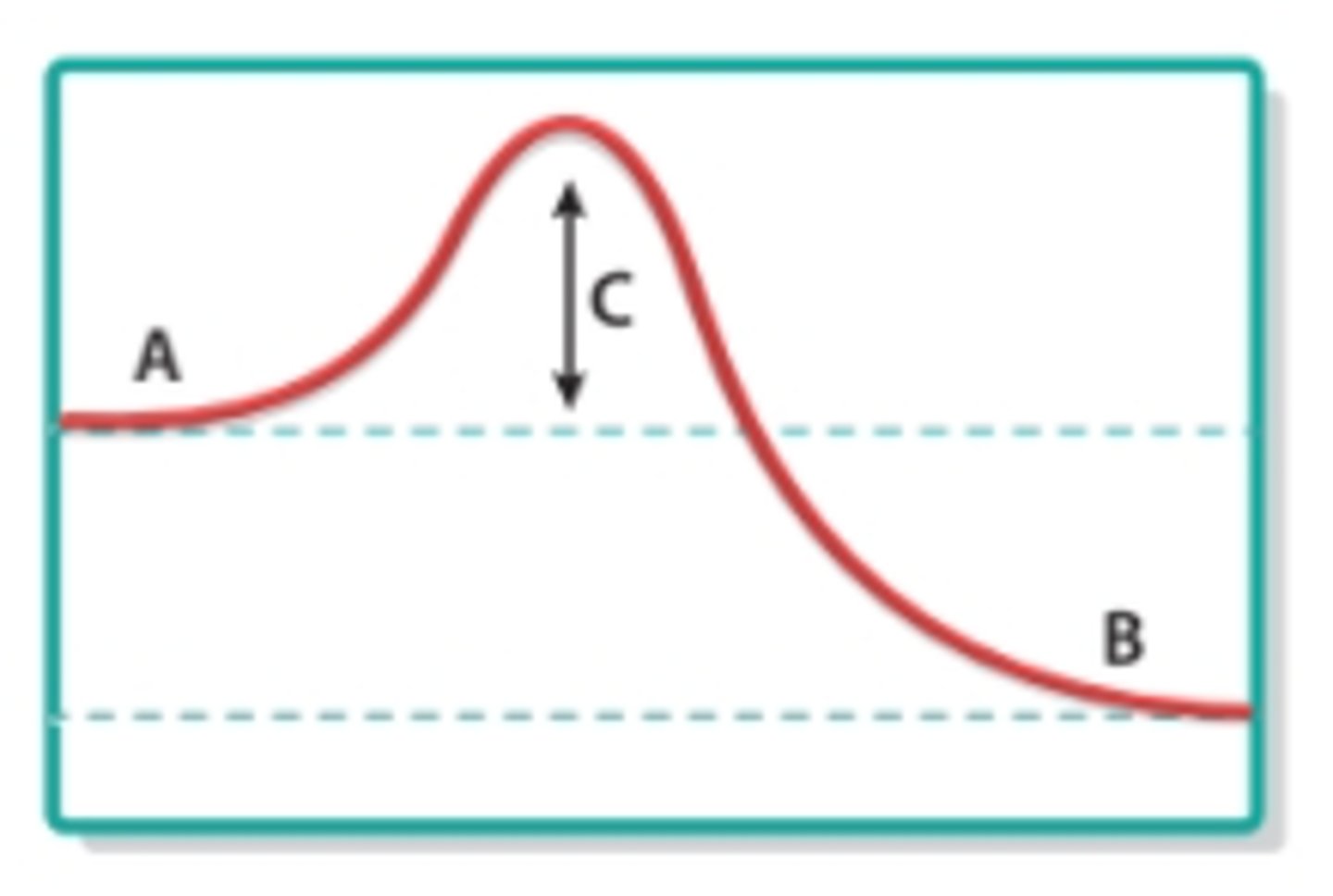
products
What does B represent in this image?
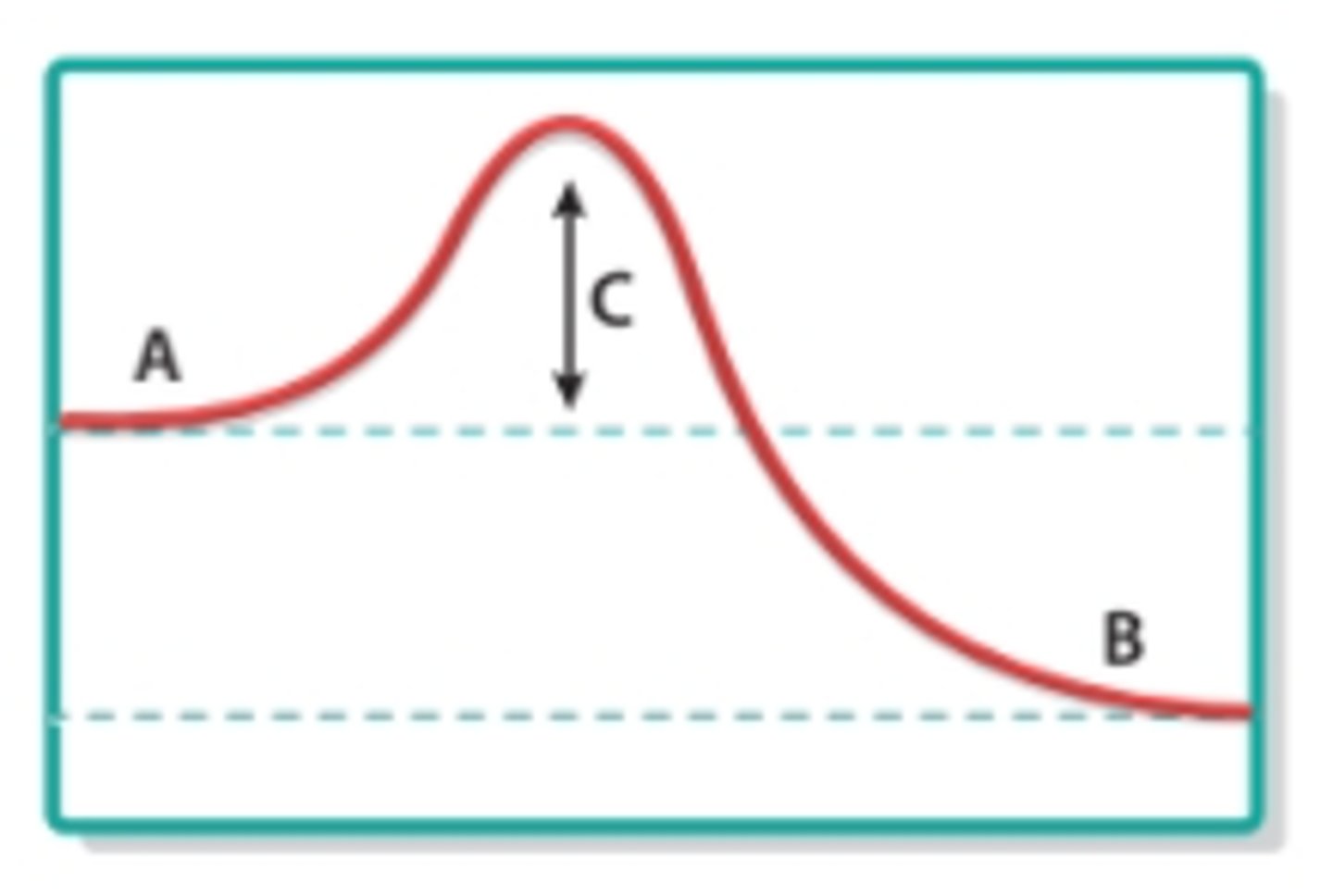
activation energy
What does C represent in this image?
the reactant particles must collide with each other, the particles have to be in proper alignment, and there must be enough energy
What does the Collision Model state must happen for a chemical reaction to occur?
surface area, stirring, temperature, concentration, catalysts
factors that affect reaction rate
concentration, temperature, pressure
factors that affect chemical equilibrium
Le Chatelier's Principle
if the system is disturbed, it will make changes to restore a state of equilibrium
double replacement
What type of reaction is this?: AB + CD → AD + CB
combustion
What type of reaction is this?: fuel + 02 → CO2 and H2O
synthesis/combination
What type of reaction is this?: A + B → AB
single replacement
What type of reaction is this?: AB + C → BC + A
decomposition
What type of reaction is this?: AB → A + B