MCAT General Chemistry - Chemical Kinetics
1/28
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
Gibbs free energy (ΔG)
determines whether or not a reaction will occur by itself without outside assistance/is spontaneous; does not necessarily mean that it will run quickly
Catalytic enzymes
selectively enhance the rate of certain reactions by a factor of 102 to 1012 over other thermodynamically feasible reaction pathways
mechanism
the series of steps in which a reaction proceeds, the sum of which gives the overall reaction
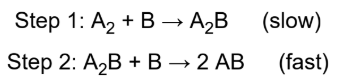
intermediate
molecule which does not appear in the overall reaction; often difficult to detect because they may be consumed almost immediately after they are formed; can be supported through kinetic experiments
rate-determining step
slowest step in any proposed mechanism; acts like a kinetic bottleneck, preventing the overall reaction from proceeding any faster than that slowest step
collision theory of chemical kinetics
the rate of a reaction is proportional to the number of effective collisions per second between the reacting molecules; orientation and energy
activation energy (Ea)/energy barrier.
The minimum energy of collision necessary for a reaction to take place; Only a fraction of colliding particles have enough kinetic energy to exceed the activation energy
rate = Z × f
where Z is the total number of collisions occurring per second and f is the fraction of collisions that are effective.
Arrhenius equation
much more quantitatively rigorous analysis of the collision theory
where k is the rate constant of a reaction, A is the frequency factor, Ea is the activation energy of the reaction, R is the ideal gas constant, and T is the temperature in kelvin.
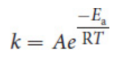
frequency factor/attempt frequency (A)
measure of how often molecules in a certain reaction collide, with the unit s–1 ; proportional to rate; Increased by Increasing Concentration
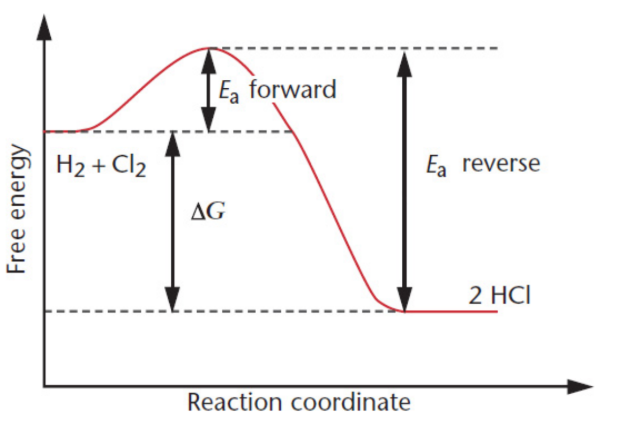
reaction coordinate
traces the reaction from reactants to products
transition state/activated complex
the old bonds are weakened and the new bonds begin to form; greater energy than both the reactants and the products and is denoted by the symbol ‡; energy required to reach this transition state is the activation energy; can either dissociate into the products or revert to reactants without any additional energy input; distinguished from reaction intermediates in that transition states are theoretical constructs that exist at the point of maximum energy, rather than distinct identities with finite lifetimes.
free energy change of the reaction (ΔGrxn)
difference between the free energy of the products and the free energy of the reactants
exergonic reaction
negative free energy change; energy is given off
endergonic reaction
positive free energy change; energy is absorbed
free energy diagram
illustrates the relationship between the activation energy, the free energy of the reaction, and the free energy of the system
Factors Affecting Reaction Rate
concentration - proportional
temperature - proportional (10°C increase doubles speed in biological systems — but optimum temperature)
medium - aqueous/organic, state of matter, polarity (polar preferred - speeds up rxns)
catalysts - proportional
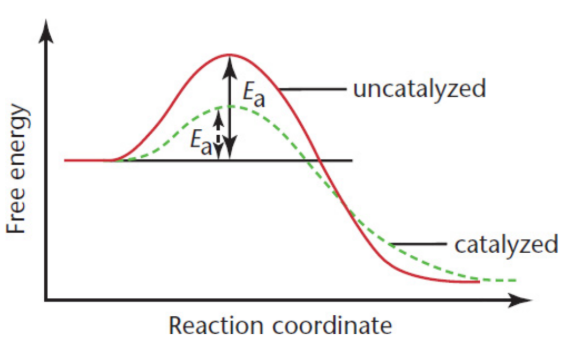
Catalysts
substances that increase reaction rate without themselves being consumed in the reaction; return to their original chemical state upon formation of the products; ONLY decreases activation energy
may increase the frequency of collisions between the reactants; change the relative orientation of the reactants, making a higher percentage of the collisions effective; donate electron density to the reactants; or reduce intramolecular bonding within reactant molecules
homogeneous catalysis
catalyst is in the same phase (solid, liquid, gas) as the reactants
heterogeneous catalysis
the catalyst is in a distinct phase from the reactants
rate
concentrations of reactants and products and their change over time
moles per liter per second or molarity per second
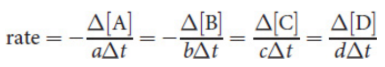
rate law
rate is proportional (by proportionality constant, k) to the concentrations of the reactants, with each concentration raised to some experimentally determined exponent; the values of x and y are almost never the same as the stoichiometric coefficients eXCEPT on the rate-determining/only step
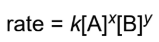
law of mass action
equilibrium constant expression
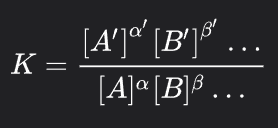
rate constant (k)
its particular value for any specific chemical reaction will depend on the activation energy for that reaction and the temperature at which the reaction takes place
Keq= k1/k-1
Experimental Determination of Rate Law
Identify a pair of trials in which the concentration of one of the reactants is changed while the concentrations of all other reactants remain constant.
Under these conditions, any change in the rate of product formation from one trial to the other (if there is any change) is fully attributable to the change in concentration of that one reactant.
Repeat this process for other reactants.
zero-order reaction
the rate of formation of product C is independent of changes in concentrations of any of the reactants; dependent on temperature/catalyst
rate = k[A]0[B]0 = k
k in M/s
t vs. [A] - negative slope = k
![<p>the rate of formation of product C is independent of changes in concentrations of any of the reactants; dependent on temperature/catalyst</p><p>rate = k[A]<sup>0</sup>[B]<sup>0</sup> = k</p><p>k in M/s</p><p>t vs. [A] - negative slope = k</p>](https://knowt-user-attachments.s3.amazonaws.com/9825fe19-48b9-494b-b181-4e68b3f84855.png)
first-order reaction
rate that is directly proportional to only one reactant
ex. process of radioactive decay
rate = k[A]1
[A]t = [A]0e–kt
k is s-1
t vs. ln[A] - negative slope = k
![<p>rate that is directly proportional to only one reactant</p><p>ex. process of radioactive decay</p><p>rate = k[A]<sup>1</sup></p><p>[A]<sub>t</sub> = [A]<sub>0</sub>e<sup>–kt</sup></p><p>k is s<sup>-1</sup></p><p>t vs. ln[A] - negative slope = k</p>](https://knowt-user-attachments.s3.amazonaws.com/51a98df6-4fe5-4b36-83aa-e8b339e7a161.png)
second-order reaction
rate that is proportional to either the concentrations of two reactants or to the square of the concentration of a single reactant
rate = k[A]1[B]1 or rate = k[A]2
k is M-1s-1
t vs. 1/[A] - positive slope = k
![<p>rate that is proportional to either the concentrations of two reactants or to the square of the concentration of a single reactant</p><p>rate = k[A]<sup>1</sup>[B]<sup>1</sup> or rate = k[A]<sup>2</sup></p><p>k is M<sup>-1</sup>s<sup>-1</sup></p><p>t vs. 1/[A] - positive slope = k</p>](https://knowt-user-attachments.s3.amazonaws.com/14da0bd4-27df-4029-bee9-f2c78127f182.png)
Mixed-order reactions
rate orders that vary over the course of the reaction
broken-order reactions
non-integer orders (fractions)