NMR Spectroscopy
0.0(0)
0.0(0)
Card Sorting
1/199
Earn XP
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
200 Terms
1
New cards
What does NMR spectroscopy probe?
It probes the absorption and emission of energy between nuclear spin energy levels when they are excited
2
New cards
Where does nuclear spin arise from?
Unpaired protons or neutrons in the nucleus
3
New cards
What does I represent?
Spin quantum number (angular momentum quantum number)
4
New cards
When number of protons and neutrons are both even…
Spin (I) is zero, these nuclei aren’t NMR active
5
New cards
If protons = odd and neutrons = even, or the other way round…. What is an example of a nuclei like this?
Spin is a 1/2 integral 1/2, 3/2…e.g.
13C p = 6 n = 7
19F p = 9, n = 10
13C p = 6 n = 7
19F p = 9, n = 10
6
New cards
Odd number of protons and neutrons?
Spin integral 1,2,3…
7
New cards
What is the problem with paramagnetic nuclei?
The magnetic moment of an unpaired electron is 1000 greater than that of nuclei, leading to additional magnetic fields leading to **large chemical shifts**, and an effective relaxation, leading to **broadened NMR signals**
8
New cards
When I = 1/2
We get good interpretable results, sharp lines
9
New cards
When I > 1/2
We can get broad signals, which are problematic. They can still be used though, and we’d need to look at the quadrupole moment of the nucleus
10
New cards
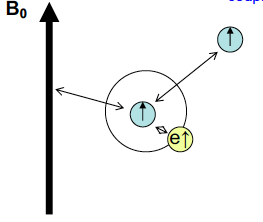
Energy levels in NMR arise from interactions of the nuclear spins with 3 things, what are they?
The spectrometer magnetic field B0
The magnetic fields created by the electrons in the system (shielding dampens external field)
The magnetic fields created by other nuclear spins in the system- which result in coupling
The magnetic fields created by the electrons in the system (shielding dampens external field)
The magnetic fields created by other nuclear spins in the system- which result in coupling
11
New cards
The gyromagnetic ratio tells us
How large the splitting is in NMR, bigger is better
12
New cards
A negative gyromagnetic ratio results in negative…
NOE enhancement, which is worth considering when decoupling NMR spectra.
13
New cards
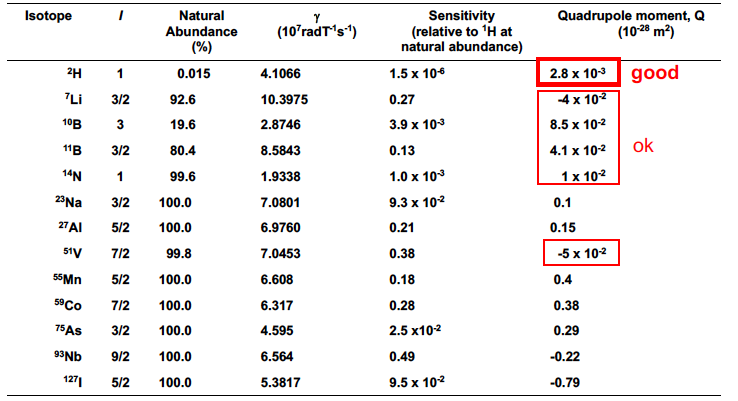
The smaller the quadrupole moment in I>1/2 nuclei, the…
Easier it is to observe splitting and transitions
14
New cards
What are some commonly used I > 1/2 nuclei?
2H, 7Li, 10B, 11B, 14N, 51V
15
New cards
A spinning nucleus possesses…, governed by the equation
Nuclear spin angular momentum P,
I is the angular momentum quantum number
\
I is the angular momentum quantum number
\
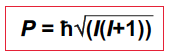
16
New cards
The spinning of a charged nucleus generates a vector, the…, governed by the equation…
Nuclear magnetic moment μ
\
\
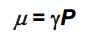
17
New cards
Again, how is the sensitivity of the detection of a nuclide dependent on gamma the gyromagnetic ratio?
Large gamma, = easy to observe , or sensitive
Small gamma = hard to observe, insensitive
Small gamma = hard to observe, insensitive
18
New cards
Equation lining magnetic moment with gyromagnetic ratio?
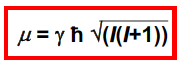
19
New cards
What is m_I? What does it represent?
The magnetic quantum number, it represents the component of the nuclear spin I along the z axis I_z
20
New cards
What are the possible values of m_I
I, I-1, … -I. To total 2I + 1 values of m_I associated with nuclear spin I
21
New cards
If a nucleus of angular momentum P and magnetic moment m is placed in a magnetic field strength of B_0 oriented along the z axis, the nuclear angular momentum orients so that

22
New cards
Different orientations of the nuclear magnetic moment will have different …. and therefore different … depending on…
Different orientations of the nuclear magnetic moment will have different
magnetic moments and therefore different energies depending on their
orientation with respect to the direction of the applied field, B0
magnetic moments and therefore different energies depending on their
orientation with respect to the direction of the applied field, B0
23
New cards
Why are only certain orientations of μ allowed to interact with the applied field B?
Each orientation corresponds to a different m_I value, this ie because of quantisation of I
24
New cards
Equation for energy of a magnetic dipole in a field?
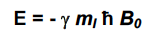
25
New cards
Selection rule for NMR transitions between spin states?
Change m_I = p/m 1
26
New cards
There are … possible transitions for a spin I nucleus, with energy…, meaning that…
2I
The same energy = -gamma hbar B0
Only one resonant frequency for the nucleus is expected, irrespective of the value of I for the nuclide
The same energy = -gamma hbar B0
Only one resonant frequency for the nucleus is expected, irrespective of the value of I for the nuclide
27
New cards
Equation for frequency of energy transition

28
New cards
What is the Larmor frequency V_L
The resonant frequency, or the frequency the nucleus precesses around the Z axis of the field, 54 degrees
29
New cards
In NMR spectroscopy, interactions with other magnetic fields from…. leads to alterations of the … and often much more than … being observed.
However, in NMR spectroscopy interactions with other magnetic fields from
the spin of other nuclides and electrons leads to alterations of the nuclide’s
resonant frequency and often to much more than a mere single resonance
being observed – Chemical Shift and Coupling!
the spin of other nuclides and electrons leads to alterations of the nuclide’s
resonant frequency and often to much more than a mere single resonance
being observed – Chemical Shift and Coupling!
30
New cards
Since frequency v depends on spectrometer field strength, how do we standardise this for nuclei?
We use chemical shift

31
New cards
Typical order of magnitude for a resonant frequency?
100 MHz
32
New cards
Do nuclear isotopes have different gyromagnetic ratios? If so, how are they related?
An isotope will have its own distinct gyromagnetic ratio, defined by
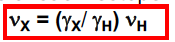
33
New cards
Why don’t we see 1H or 195Pt nuclei signals in a 31P NMR spectrum?
Transitions are induced between different energy levels by irradiating with a superimposed field B1 of the correct quantum energy, so we tune to the nuclide of choice!
34
New cards
NMR signals strength depends on
The population between the ground and excited states
The two processes occur at the same energy
therefore the intensity of the observed NMR signal depends on the
difference between the numbers of absorption and emission
processes occurring – net movement
The two processes occur at the same energy
therefore the intensity of the observed NMR signal depends on the
difference between the numbers of absorption and emission
processes occurring – net movement
35
New cards
What does net movement between spin states depend on?
The population difference given by Boltzmann statistics, since the probabilities of emission and absorption are equal.
36
New cards
N_upper/ N _lower = ….
Usually a very small population difference!
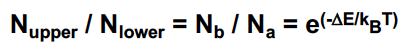
37
New cards
The observed signal is proportional to
N-a - N_b
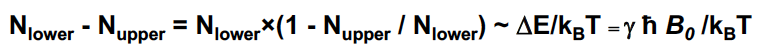
38
New cards
If the populations in each state are exactly equal, what happens
Saturation, no signal is observed
39
New cards
Nuclides with low gyromagnetic ratios tend to give…
Sensitivity of a nucleus is proportional to? For a fixed B
Sensitivity of a nucleus is proportional to? For a fixed B
Weaker signals
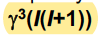
40
New cards
Relative receptivity is given relative to… What equation form do we use?
1H or 13C
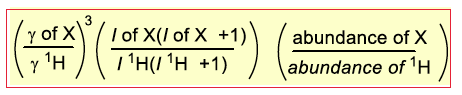
41
New cards
How else can we increase the sensitivity of an NMR experiment? (3 ways)
Use a stronger magnet in the spectrometer, by increasing B_0 the Boltzmann distribution becomes more favourable
We can also lower sample temperature, which increases population difference to give a stronger, but broadened signal
Use more concentrated samples
We can also lower sample temperature, which increases population difference to give a stronger, but broadened signal
Use more concentrated samples
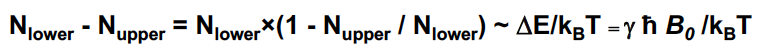
42
New cards
Describe the FT NMR experiment, why do we do it?
We use pulsed NMR to irradiate all spectral frequencies at once, then detect all the resonances at the same time.
When the states relax they emit radiation, which comes out as a FID signal, converted to a real spectrum by Fourier Transform techniques.
It is very time efficient and allows “rapid multiple passes” so a good signal to noise ratio can be established.
When the states relax they emit radiation, which comes out as a FID signal, converted to a real spectrum by Fourier Transform techniques.
It is very time efficient and allows “rapid multiple passes” so a good signal to noise ratio can be established.
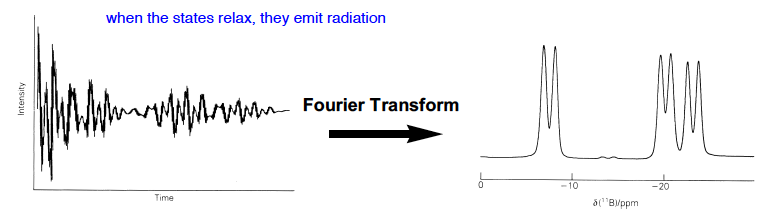
43
New cards
Chemical shift is defined with respect to…
The nuclei in a reference compound, whose chemical shift is usually given the arbitrary value of zero.
44
New cards
The convention for +ve and -ve chemical shifts, relating to shielding, direction, field frequency?
\+ve shift, higher than standard frequency, indicating deshielded environment, and is low-field. (left)
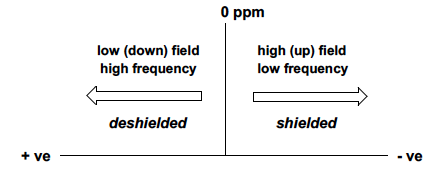
45
New cards
Coupling to n chemically equivalent nuclei gives rise to….
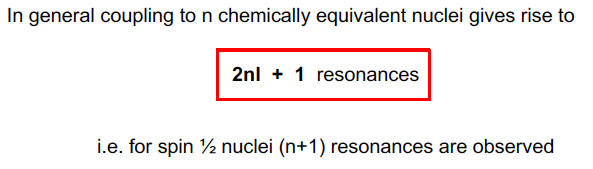
46
New cards
Different spin states result in
Different energies/frequencies/shifts
47
New cards
In a coupling tree, we should start with
The higher coupling constants
48
New cards
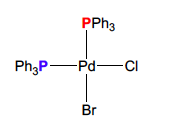
How many peaks does this compound have
2 P31 resonances, since 2 different chemical environments
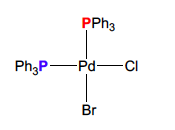
49
New cards
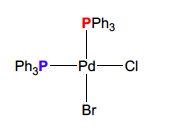
What coupling pattern should arise from this compound?
2 doublets with equal intensity
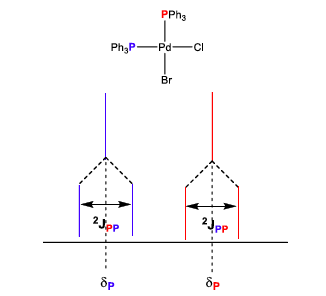
50
New cards
What is a first order splitting pattern?
Like the one above, where
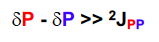
51
New cards
When do second order effects occur, and what do I expect to see?
When the difference in shifts delta P- delta P is similar to the coupling constant 2J_pp
We expect to see roofing distortions, in an A=B system. If it becomes A2, we only see a singlet and no coupling
We expect to see roofing distortions, in an A=B system. If it becomes A2, we only see a singlet and no coupling
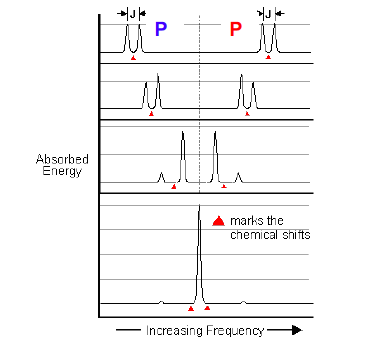
52
New cards
What 3 big factors affect the magnitude of the coupling constant J?
%S character, higher like SP is better than SP3. Nuclear spin interacting with electrons needs electrons close to the nucleus, s electrons have a non-zero probability of being at the nucleus.
Stereochemistry, trans couplings are stronger, since we need good alignment of bonds
Number of bonds between nuclei, 1J is 10x higher than 2J, which is similar to 3J
Stereochemistry, trans couplings are stronger, since we need good alignment of bonds
Number of bonds between nuclei, 1J is 10x higher than 2J, which is similar to 3J
53
New cards
What is broad band decoupling, and why do we use it?
31P NMR spectra of some phosphines get complicated multiplets due to H-P coupling
We simplify them by broad band decoupling the proton frequency, by irradiating the 1H frequency as well as 31P, so there is no coupling to protons
We simplify them by broad band decoupling the proton frequency, by irradiating the 1H frequency as well as 31P, so there is no coupling to protons
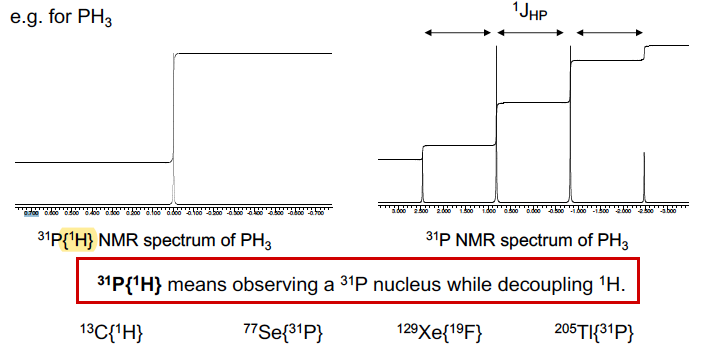
54
New cards
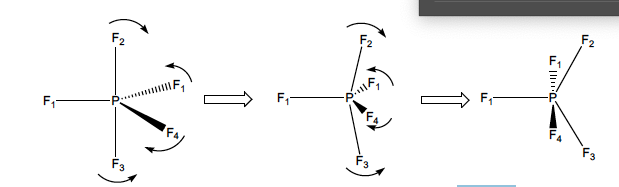
Given the molecule PF5, why do we observe 1 signal at RT?
The exchange of fluorine NMR states is too fast for the NMR timescale (fluxionality)
If we wanted to see it, then we could cool the sample down.
If we wanted to see it, then we could cool the sample down.
55
New cards
What are isopotomers?
Compounds with isotopes of nuclei resulting in slightly different chemical shifts
56
New cards
What dilute I = 1/2 nuclei could we expect to display satellites?

57
New cards
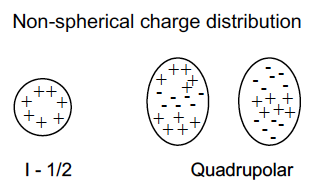
What does quadrupole moment arise from?
Electric field gradient at nucleus from uneven charge distribution
58
New cards
What do quadrupoles do to lines?
The electric field gradient interacts with the magnetic field, leading to rapid relaxation of nucleus
Peak width increases with 1/T2
Nuclei with I > 1/2 possess a quadrupole moment Q
• an electric field gradient that interacts the nuclear magnetic moment
• results in fast relaxation of the nuclei, which scrambles energies of the spin
states
• meaning that NMR spectra are broad (expressed as width at half height Dv1/2)
• coupling is often not resolved
• line widths proportional to Q^2
Peak width increases with 1/T2
Nuclei with I > 1/2 possess a quadrupole moment Q
• an electric field gradient that interacts the nuclear magnetic moment
• results in fast relaxation of the nuclei, which scrambles energies of the spin
states
• meaning that NMR spectra are broad (expressed as width at half height Dv1/2)
• coupling is often not resolved
• line widths proportional to Q^2
59
New cards
Do we want large or small quadrupole moments?
Small
60
New cards
What is the name of the process where we see broad lines due to rapid relaxation rates?
Heisenberg broadening
61
New cards
When can we still use quadrupolar nuclei for NMR?
When we have a highly symmetrical environment like octahedral or tetrahedral
These give line widths as if I = 1/2
Less symmetric increases relaxation rate, defining the energy levels poorly for broad signals.
These give line widths as if I = 1/2
Less symmetric increases relaxation rate, defining the energy levels poorly for broad signals.
62
New cards
Why are couplings washed out in quadrupolar nuclei?
Poorly defined energy levels
63
New cards
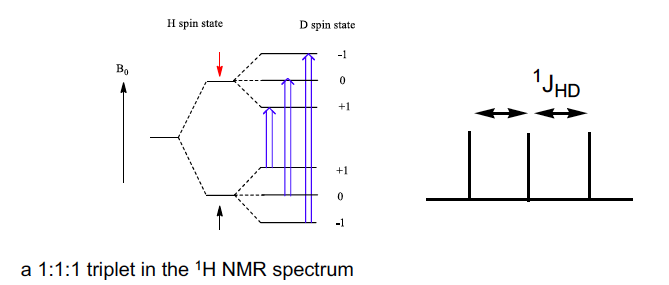
How does coupling in 2D or 6Li differ from a triplet from 1H (I= 1/2)
I = 1, so 3 peaks BUT all at same intensity since the 3 possible spin states M= 1,0,-1 all have equal probability
1:1:1 triplet
1:1:1 triplet
64
New cards
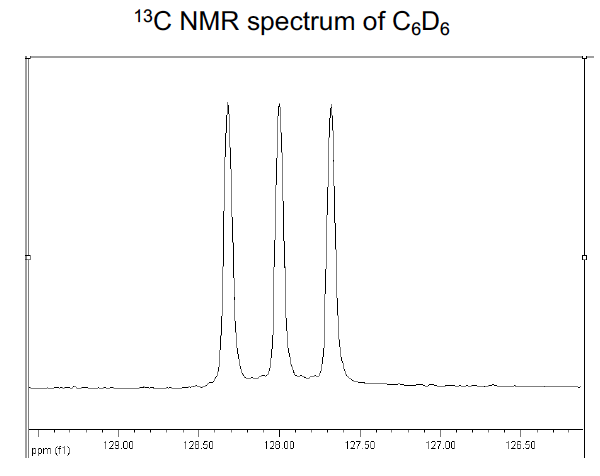
Predict the 13C NMR of C6D6
65
New cards
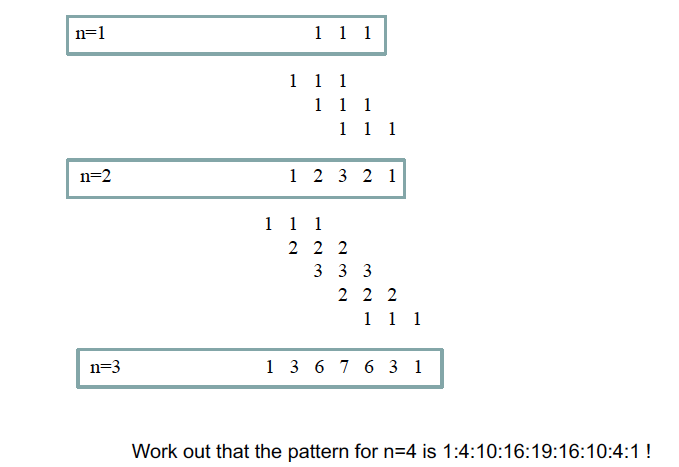
Does Pascal’s triangle hold for I > 1/2 nuclei
No
66
New cards
How do work out the coupling pattern for n nuclei of I = 1?
67
New cards
Signal increase by factor … for N scans
Noise increases by factor…
Noise increases by factor…
Signal by N
Noise by sqrt(N)
Noise by sqrt(N)
68
New cards
For N scans, signal to noise ratio increases by
sqrt (N)
69
New cards
If we have 1/N concentration, how many more scans do we need to get the same quality spectrum, at same width
N^2 scans
70
New cards

In this example, how many more scans do we need to do for 1 to have the same S/N as the other?
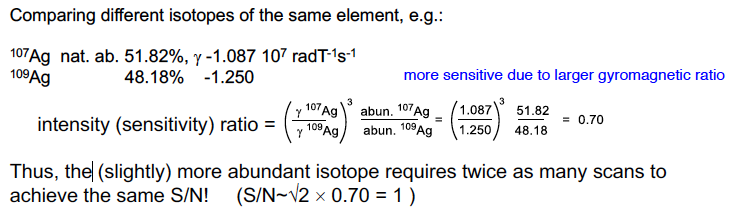
71
New cards
Why is there no net magnetisation for molecules in solution?
The molecules are randomly tumbling, in the absence of an applied field, all the nuclear magnetic moments will be randomly oriented
72
New cards
Heisenberg uncertainty principle in FT NMR
By limiting irradiation time, we have less uncertainty in time and energy, so length of pulse is uncertainty over time.
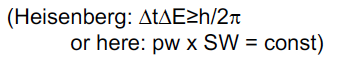
73
New cards
Shorter pulse on range of frequencies
Shorter pulse = larger range of frequenciess
74
New cards
Nuclear spin transitions cause
A displacement of M from the z’ axis towards the y’ axis by an angle theta, the pulse/tip angle
75
New cards
What determines the pulse angle through which M is tipped?
The strength/time length of the excitation pulse B1
76
New cards
After the pulse has been applied, M…
precesses about the z’ axis until it returns to the pre-pulse state, relaxing back
77
New cards
The observable NMR signal is
the projection of this precession onto the x’-y’ plane. Since the receiver coil is oriented along the y’ axis, the intensity of the signal depends on the amount of magnetisation in the y’ direction, My
78
New cards
Maximum signal intensity if observed at, and zero intensity expected at
Mzximum at theta = 90 degrees
Zero at 0 degrees
Zero at 0 degrees
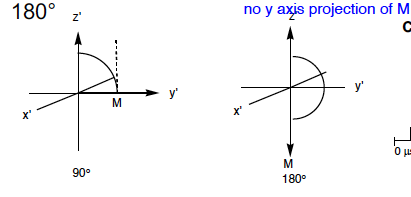
79
New cards
What happens to energy and magnetisation during relaxation?
The magnetisation begins to revert back to its eqm state, the excited nuclear spins lose energy and drop to the GS
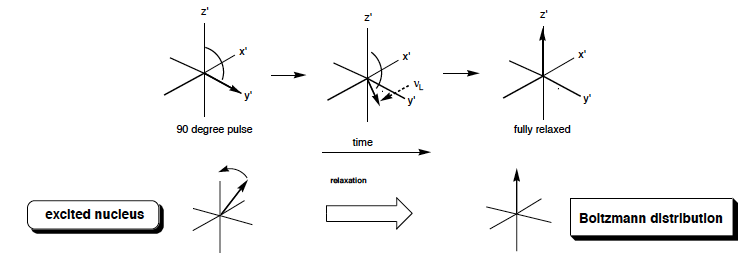
80
New cards
Timescale of relaxation
seconds to hours
81
New cards
Generally, relaxation occurs by…
transfer of magnetisation to other dipoles
82
New cards
What is dipole-dipole relaxation?
Dipole-Dipole relaxation occurs when the sample contains other nearby magnetic dipoles that can interact with the nuclear magnetic moment e.g. nuclei or electrons
83
New cards
Effectiveness of relaxation depends on and is proportional to, so for two nuclei, the higher … will give faster relaxation.

84
New cards
What determines how fast a nuclear magnetisation relaxes to equilibrium magnetisation?
The number and distance to neighbouring dipoles
85
New cards
In the absence of more effective relaxation mechanisms, relaxation time will depend on…
number of nearby H atoms (due to high g ratio) and is dominated by directly bonded H CH3 > CH2> CH >>> C
86
New cards
Long relaxation times occur with (4)
Low viscosities, high temperatures and small molecular masses and concentration.
\
\
87
New cards
Are long relaxation times desirable?
Yes
88
New cards
Dipole dipole interactions occur by 2 clear mechanisms, what are they?
Spin-spin relaxtation T2
Spin-lattice relaxation T1
Spin-lattice relaxation T1
89
New cards
The shorter the relaxation times, the … the signals
The shorter the relaxation times, the
broader the signals
broader the signals
90
New cards
T1 vs T2 in isotropic media
T1 = T2
91
New cards
Equation for T2
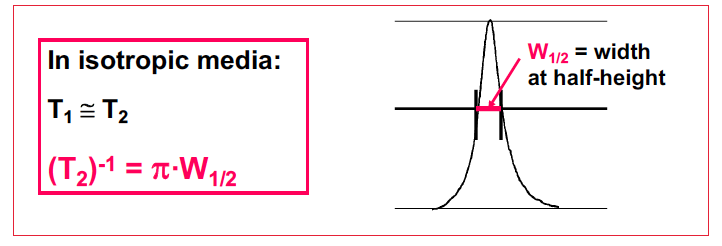
92
New cards
When can we expect effective relaxation and broad lines? (5)
Viscous media
Large molecular mass (proteins)
Low temperature (more viscous)
Quadrupole moments with lower than cubic symmetry
Nuclei interact with paramagnetic centres (paramagnetic shift reagents)
Large molecular mass (proteins)
Low temperature (more viscous)
Quadrupole moments with lower than cubic symmetry
Nuclei interact with paramagnetic centres (paramagnetic shift reagents)
93
New cards
In Spin-Spin relaxation T2, a nucleus of 1 atoms…
A nucleus of one atom imparts or exchanges its energy (magnetisation) to
another surrounding nuclei resulting in no overall change in spin
populations
another surrounding nuclei resulting in no overall change in spin
populations
94
New cards
Spin- spin relaxation corresponds to the magnetisation...
Fading away in the x’-y’ plane
95
New cards
Longer values of T2 give
Narrow line widths
W1/2 = 1/ pi T2
W1/2 = 1/ pi T2
96
New cards
Describe Spin-Lattice relaxation T1
Decay of magnetisation by the tipped nucleus returns to its normal state by exchanging magnetisation energy with surroundings- the lattice. It corresponds to the growing back of magnetisation along the z’ direction
97
New cards
What is T1 important for>
It determines how frequently we can pulse the nucleus since it is the slower process when compared to T2, in the extreme case they’re the same
98
New cards
Describe the the process to measure T1
Inversion recovery, we probe signal inetnsity after time tau. We plot intensity against tau, and use the model to find T1
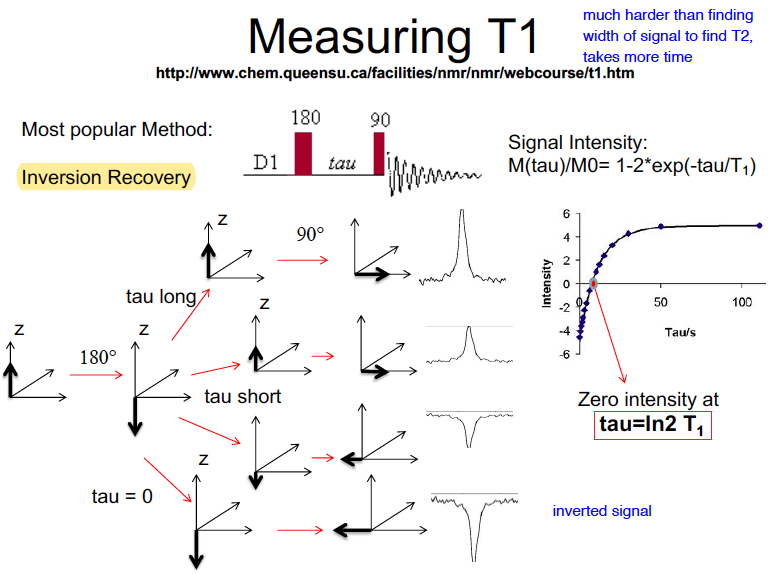
99
New cards
At zero intensity, tau =
ln2 (T1)
100
New cards
What 2 things does quadrupolar relaxation depend on (when I>1/2)
Nuclear quadrupole and electric field gradient of nucleus