Intermediate Filaments
1/56
Earn XP
Description and Tags
Cell Biology
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
How many classes of intermediate filaments are there?
5 classes
Light Description: List the IF classes, their proteins, and their locations
Class I: Acidic Keratins, epithelial cells
Class II: Basic keratins, epithelial cells
Class III:
Vimentin, Mesenchymal cells
Desmin, muscle cells
Glial Fibrillary Acidic Protein (GFAP), glia
Peripherin, PNS neurons
Class IV: Neurofilament proteins (NF-L, NF-M, NFM, NF-H, alpha-internexin), neurons
Class V: Nuclear lamins (A, B, C), nuclear lamina
Describe the structure of intermediate filaments: monomer to Dimer to TETRAMER to Unit Length Filament
Intermediate filaments: made of long alpha coiled-coil loops with a middle intermediate, not globular
They intertwine and stack on each other longitudinally (unlike actin and mts)
Dimer: Organizes C-Terminuses together
Tetramer: Two dimers joint at opposite ends
ULF (intermediate): 32 monomers (8 tetramers) bind together antiparallel to ensure the entire filament is nonpolar
Full IF: These bind together to form chemically identical ends.

How are IF polymers different from microtubules and actin filaments?
There’s no NTP binding/hydrolysis
Non-polar/chemically identical ends
No motor proteins travel along IFs
Initial nucleation is slower
Subunits exchange along the length instead of along the edge
What is vimentin?
Another type of intermediate filament that fits in between intermediate filaments

What is the elementary piece of a Intermediate filament? What is it’s analogue in actin and mts?
IF: the tetramer
Actin: one globular protein
MT: Alpha-Beta tubulin dimer
How do you depolymerize intermediate filaments
Phosphorylation: by introducing phosphates anywhere along the length of the filament causes disassembly
Why is it necessary that IFs depolymerize depolymerize during mitosis?
they’re too interconnected to everything and too stretchy and adaptable to pulling stress
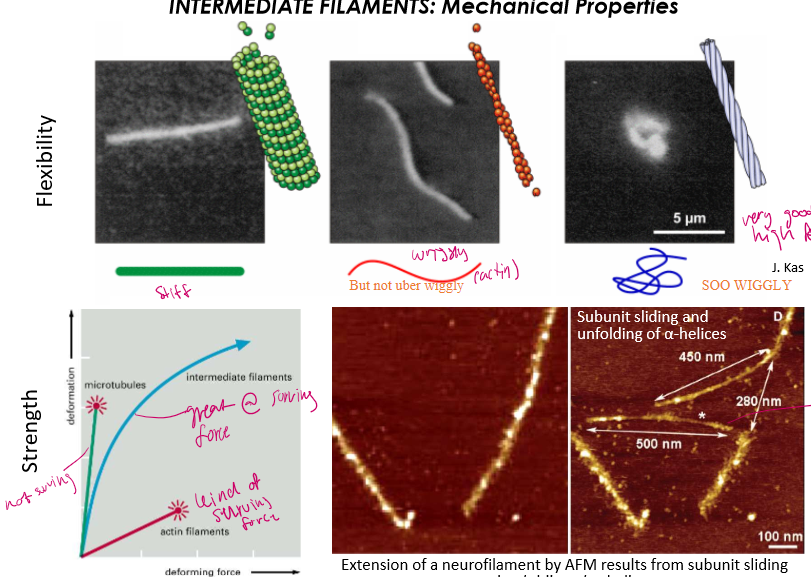
Order the flexibility of cytoskeletal strflexibleMostucturers from most felxible to least
Most: IFs: will not break easily under stress, rather it will extend
Middest: Actin: wiggly but not super strong
Lowest: is microtubule: girl will snap like a pretzel the moment you pull
Who is the Plakin family
These are cross-linkers. The region next to the n-terminus is full of alternatively spliced regions that can create different structures for binding. They allow IFs to integrate into the entire cytoplasm
Full of famous girls like plectin (a ubiquitous protein), BPAG & desmoplakin (both are epithelial cell localized)
Who is plectin? Describe her structure
Plectin is a major IF that links longer IFs to actin
Isoform-specific domain=N-terminus: localized plectin to a particular organelle or cell
Actin binding domain: links IF to actin
Variable alternative splicing creates different binding structures
Coiled-coil domain: stretchy tail of plectin
IFBD: Intermediate filament binding domain.

What is the MAIN FUNCTION of IFs?
Increasing the mechanical strength of cells and tissues
Describe the structure and composition of Class I & II IFs
Acidic Keratins and Basic Keratins:
They dimerize with each other in epithelial cells, and then form acid-base tertamers

What do keratin intermediate filaments attach to
To the nuclear envelope and to desmosomes (cell-cell adhesion structures between epithelial cells)
How is keratin anchored at intermediate filaments?
Intermediate filament←c-terminus of desmoplakin←plakoglobin & plakophilin (adaptor proteins)← bound to specialized cadherins that hold cells together

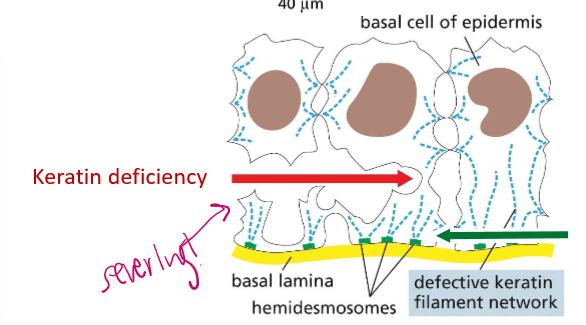
What is a hemidesmosome?
The region binding a cell and extracellular filaments. It’s not mediated by adherins, but by integrins.

What happens if BPAG or plectin mutate in their location?
If they mutate in the epithelia, the epidermis will detach from the lower layers of skin, creating terrible blisters. Because they bind to laminins (laminal proteins), they are bound by intergrin.
List the Class III IFs and their localizations
Vimentin: Mesenchymal and undifferentiated cells (but can also be expressed in differentiated cells… WHEN THEY’RE CANCEROUS!)
Desmin: muscle cells, localized by alpha actinin, so in z-disks
Glial fibrillary acidic protein (GFAP) in glia
Peripherin in PNS neurons
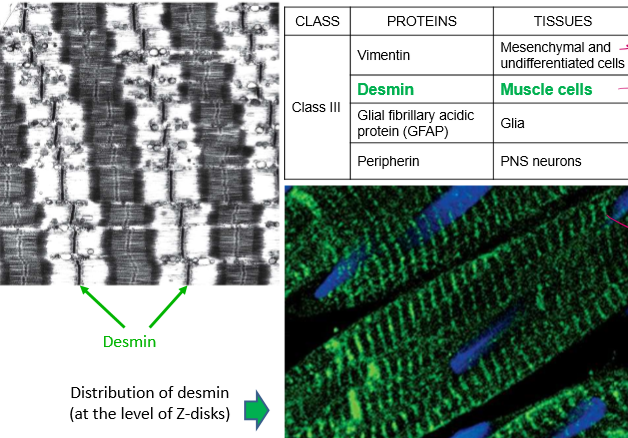
What does desmin do?
They’re IFs that wrap around myofibrils, in order to organize the sacromere. They can wrap z-disks and mitochondria, for example, to keep them away from damaging contractile filaments and keep an ATP source close
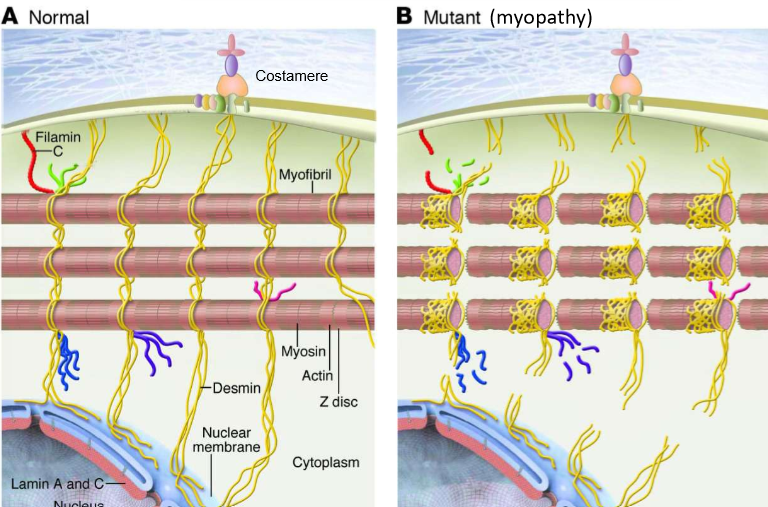
What are costamers?
Linkers of IFs to the extracellular matrix. They link to DGCs: dystrophin-glycoprotein complexes. They serve as cytoplasmic attachement sites

How do mutations in desmin affect muscle cells?
A loss can lead to muscle cells over-contracting in myofibrils, leading to tears in filaments and damage
Name and localize Class IV IFs
Neurofilament proteins (NF-Light, NF-Medium, and NF-Heavy), localized in neuronal tissues
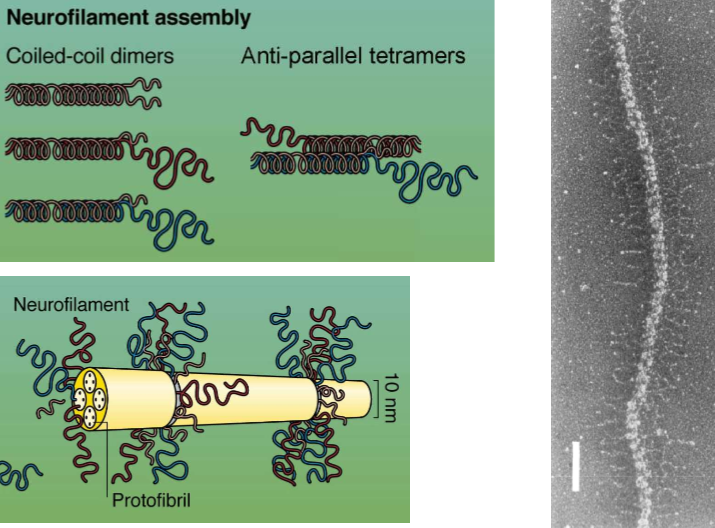
HOW do neurofilament proteins polymerize
The long tails form coil-coil dimers. Every dimer must be at least 50% NFL. NFLs can dimerize with themselves, but the other NFs must join
Describe the structure of NFs
All three have n-terminus heads and coil rod sections of the same time.
C-terminus heads have tails of light, medium, and heavy length that all very.

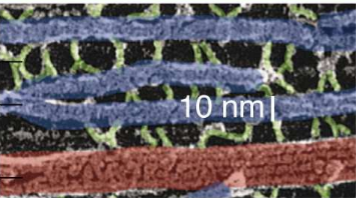
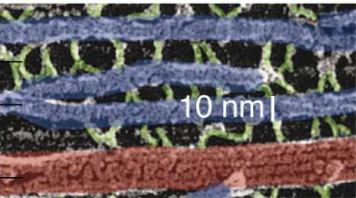
What do NFs do thanks to their structure?
Their coil tails dimerize and then form anti-parallel tetramers to create lateral projections in neurofilaments.
They’re surrounded by protofibrils, and the tails that stick out interact with other filaments. They can thus form bridges with other NFs
What are the functions of NFs
Their stretch and recoil provide structural integrity to the non-stagnant axons that are deformed by muscle acfivity
Increased IF density strengthens and improves the conduction of electrical signaling
They thus define the diameter of axons
They strengthen long and thin neuronal processes
They integrate neurons to skin and extracellular surfaces.
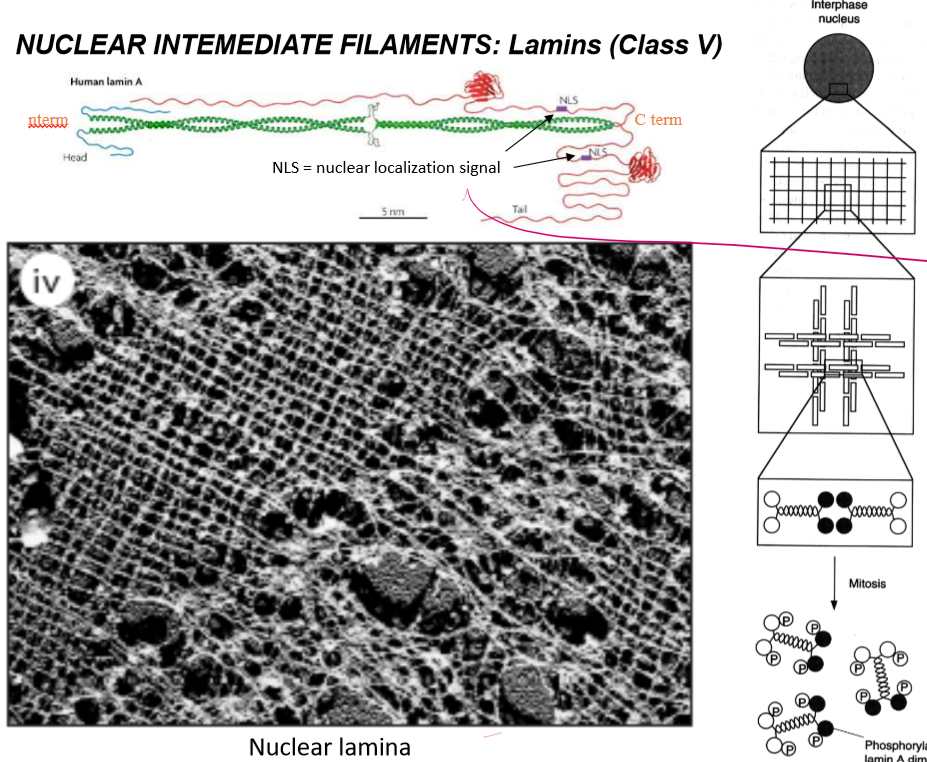
Describe and localize the Class V IFs
Lamins: localized around the nucleus
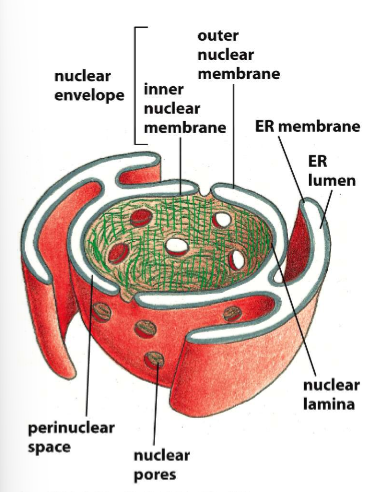
Describe the connectivity between the ER and the nuclear membrane.
The outer nuclear membrane is continuous with the ER membrane like some sort of wrap, but the inner nuclear membrane is separate and lined with lamina to bind that to the cytoskeleton
How do lamins get localized into the nucleus?
Via nuclear localization signal on the very long C-terminus. This signals delivery through the treacherous nuclear pore.
How are lamin tetramers regulated?
They’re disassembled by phosphorylation into their dimers to allow the nuclear envelope to dissolve during mitosis.
Describe the structures of lamins?
They dimerize as long coil-coils with intermediate space, and have long streamy c-terminal tails that house nuclear localization signals. Their dimers form tetramers that for netlike webs in the inner nuclear membrane
Who are the binding partners of lamina?
Lamina bind to SUN-domain intermembrane proteins, who are bound to KASH-domain intermembrane proteins in the perinuclear space.
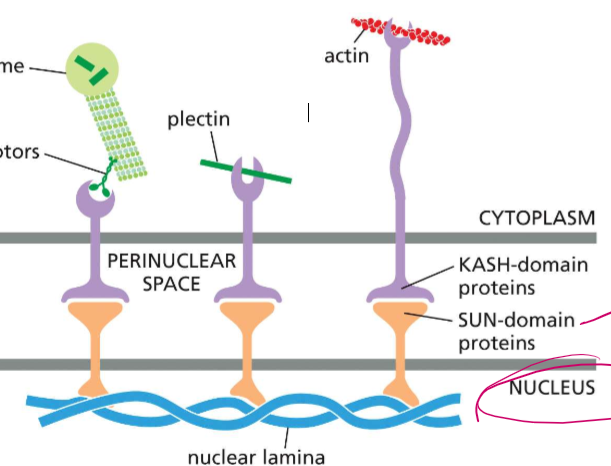
Who are the binding proteins of KASH-domain proteins?
SUN-domain proteins (who are bound to nuclear lamina or chromosomes
In the cytoplasm, KASH-domain proteins can specialize and bind to
motor proteins cargo domains, who’s motor heads are BOUND to microtubules or centrosomes
plectin (for stabilization)
actin

BRIEF functions of lamina
nucleus stability
nucleo-cytoplasmic coupling
distribution of nuclear pores
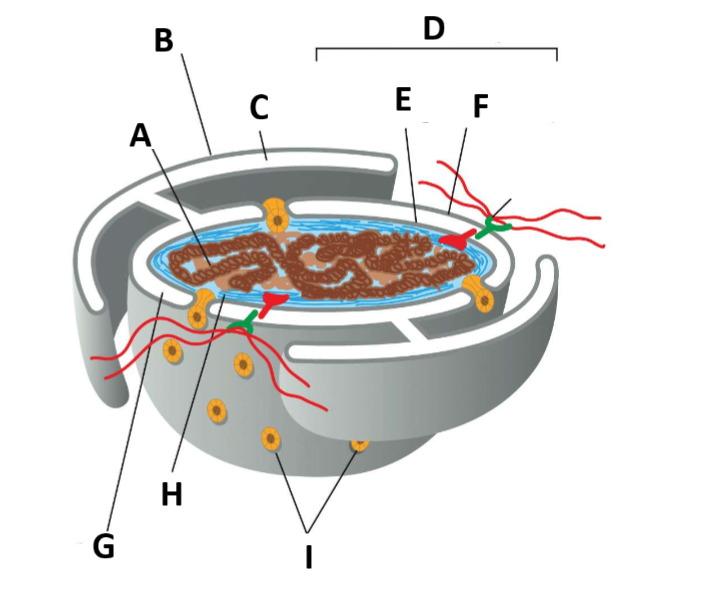
Yk we needed a label question…
A - Chromatin
B - ER membrane
C - ER Lumen
D - nuclear envelope
E - Inner nuclear membrane
F - Outer nuclear membrane
G - Perinuclear space
H - nuclear lamina
I - Nuclear pore complexes
J - Links from lamina to cytoskeleton

Describe the VAGUE structure of the nuclear pore
They have 8-fold pyramidal symmetry to form the basket
actual complexes that build into the nucleus
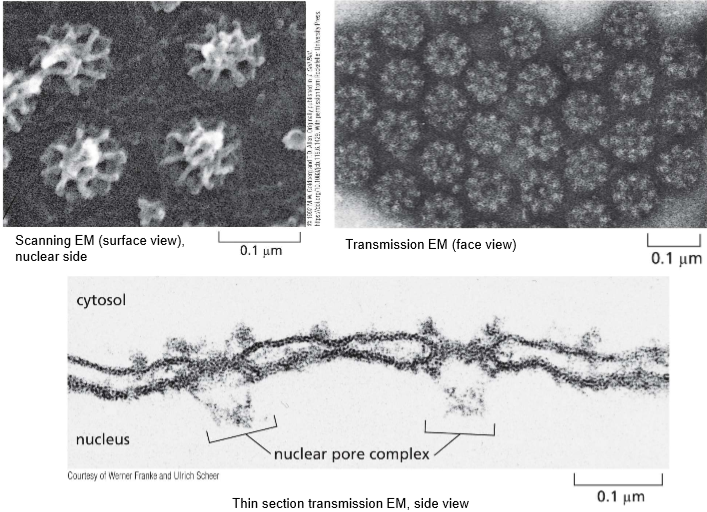
All nuclear pores are built the same! SO HOWWW are they built
The nuclear basket extends into the inner nucleus
Cytosolic fibrils extend out into the cytosol
The disordered region of channel nucleoporins
scaffolding nucleoporins hold them to the side
The nuclear envelope holds the width of the pore
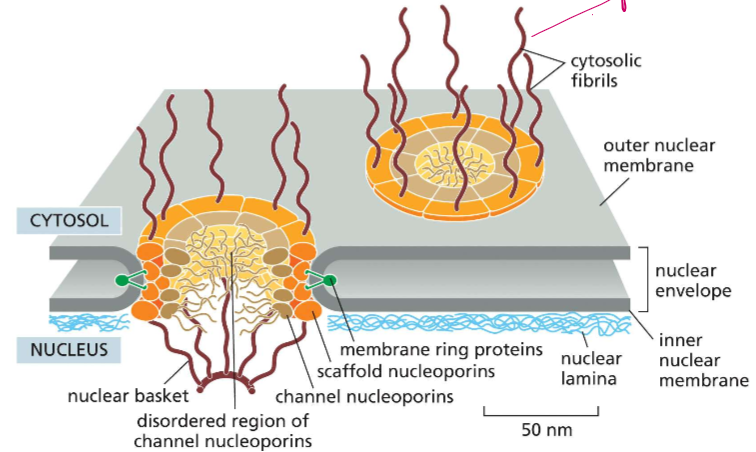
The nucleoporin is DENSE: let’s talk about light descriptions of objects rolling in: how big are they
It’s a maze molecules must travel and fit through. No molecules over 40nm in length can pass through freely
What is the nucleus import signal?
A short, positively charged
Lys, Lys, Lys, Arg, Lys (KKKRK)
What is the export nuclear code?
Shortish, hydrophobic sequences 2, 3, and 3 random amino acids apart.
Met, X,X, Leu, X,X,X, Leu, X, X, X, Phe (MXXLXXXLXXXF)

How do mutations in localization signals affect movement?
Even just one mutation can prevent nuclear localization.
Describe nuclear localizations in terms of T-Antigens
T-Antigens stop DNA transcription and start viral transcription. They’re from viruses, and they’re the mechanism by which they hijack the cell’s DNA replication. For those proteins to even enter the nucleus, however, they require the nuclear import signal of KKKRK (or several positive amino acids in a row) to enter through the nuclear pores.
What are kariopherins?
Nuclear transport receptors of the Importin Beta family: they bind to nuclear localization signals on cargo proteins and then carry them through nuclear pores. If a protein does not have her own kariopherin, an adaptor protein with a localization signal is then carried into the pore via karioprotein while being attached to cargo.


How do cargo proteins know to release cargo in the nucleus if they’re not pH mediated?
They’re Ran-GTPase gradient mediated
inactive Rans exists in the cytoplasm, while active Rans exist in the nucleus
GEF for RanGTPase is always bound to chromotin, and can only change Ran’s comformation when it gets in
GAP for RanGTPase is always bound to the nucleofibrils of the cytoplasmic side of the nucleoporin.

Why can’t the nucleus be pH mediated
Hydrogen protons are so motherfreaking small. As such, the protons would just equilibrate through the nucleopores.
Who are exportin and importin?
Kariopherins (nuclear transport receptors) that IMPORT INto the cell cargo proteins and export old proteins out
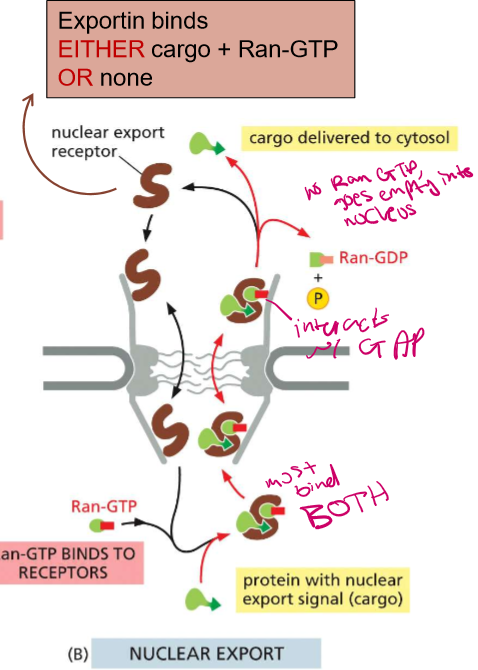
Describe (in detail) Ran-Dependent directionality of nuclear transport into the nucleus
Importin binds to EITHER cargo or Ran-GTP
In the nuclear space, Ran-GTP bound outcompetes the cargo (its at high concentration) and is then attached to importin to leave via the pore
There, Ran encounters its GAP on the nucleoporin fibrils and exchanges it with GDP and stays in the cytoplasm
Ran-GDP bound cannot cannot bind to importins.
There is No Ran-GTP-Bound GTPase in the cytosol

Describe (in detail) Ran-Dependent directionality of nuclear transport into the nucleus
Exportin must bind to BOTH Ran-GTP-Bound GTPase and cargo, or NOTHING, before carrying them out to the cytoplasm
When the complex then goes outside of the nucleus, it will interact with the GAP on the nucleoporin fibril.
Cargo gets delivered to the cytosol and the Ran, which cannot bind without being bound to GP
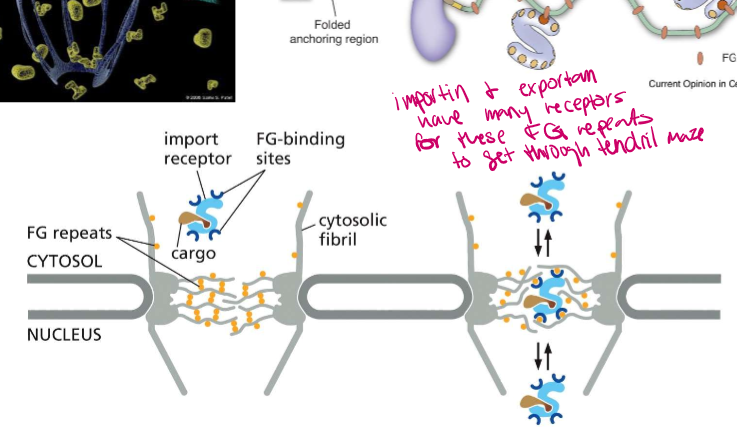
What are FG repeats?
Phenylalanine-Glycine repeats on the nucleoporincomplex fibrils. They bind to FG-binding sites on importin and exportin to help them get through the nucleoporin maze
What cation signals an immune response from the nucleus.
Calcium
Who does calcium activate in beginning an immune response?
calcineurin, a protein phosphatase
Who is NF-AT
Nuclear factor of activated T-cells: a transcription factor who intitiates gene transcription as an immune response.
What does NF-AT, (nuclear factor of activated T-cells) look like in it’s inactive form?
A protein that is bound to 3 phosphates with it’s nuclear export signal exposed while it’s in the cytosol
Who does calcineurin dephosphorylate?
NF-AT (nuclear factor of activated T-cells). This dephosphorylation exposes its nuclear import signal while the calcineurin then binds to the nuclear export signal.

Describe the full process for shuttling proteins in an immune response:
High Calcium ion concentration activates calcineurin
Calcineurin dephosphorylates NF-AT and binds to the nuclear export signal, blocking it
The nuclear import signal is also then exposed, allowing NF-AT to bind to importin and enter through the nuclear pore
It will then activate transcription
Calcineurin will then dissociate in the low calcium concentrations, exposing the export signal and phosphorylating NF-AT again for transport
Exportin can then bind and take it out

Cyclosporine A inhibits calcineurin. How does this inhibit the shuttling protein pathway.
Inhibition prevents the dephosphorylation of the NF-AT, which will therefore prevent its entrance back into the nucleus. This will prevent gene transcription and whatever cell growth we wanted to create.
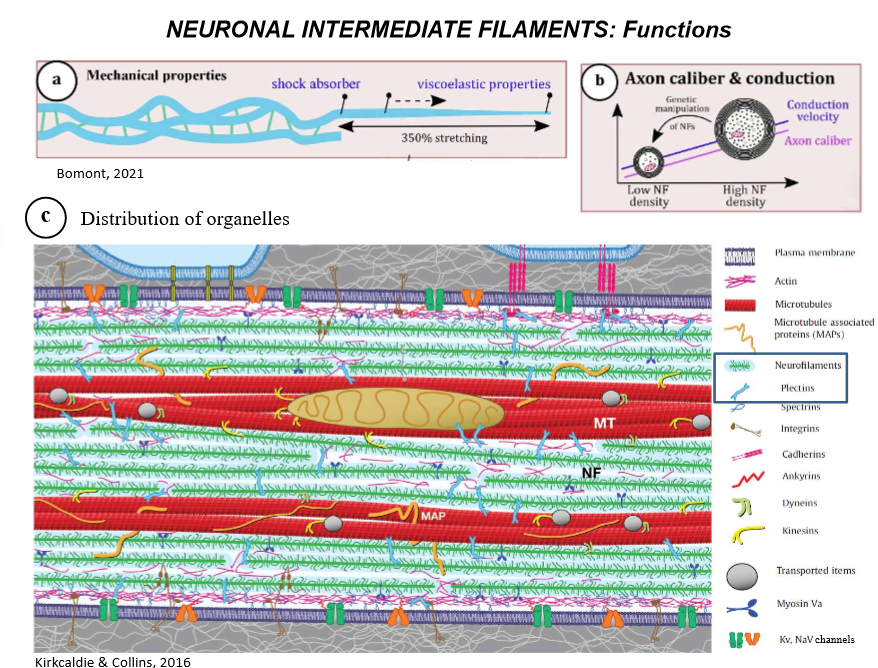
What are the FUNCTIONS of neurofilaments?
shock absorber (for damage when they move)
providing elasticity to the neuron and strengthening the long length
Appropriately distributing neuronal organelles along the axons
conducting electrical signals
integrating the neuron to extracellular surface ie skin
