Conformation of saturated rings
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
what is ring strain energy
extra energy a molecule has by virtue of being constrained to a ring, determined from heats of combustion
what are small rings
3-4 members, ~25 kcal/mol, e.g cyclopropane, aziridine
what are normal rings
5-7 membered, ~5 kcal/mol, include piperidine and cyclopentane
what are medium rings
8-14 membered, 5-15 kcal/mol include cyclooctane and cyclononane
what are large rings
>14 membered, very low energy due to being almost linear, include cyclohexadecane
what are contributions to conformational energy unique to rings
baeyer (angle) strain and transannular strain (medium rings only)
what is baeyer (angle) strain
atoms are distorted from their ideal bond angle in order to form a ring
what is transannular strain
only effects medium rings (8-14 membered), substituents point at each other across the ring leading to additional steric strain
describe the conformation of cyclopropane
all Cs in one plane, all C-C bonds are eclipsed so has high pitzer strain, much smaller bond angles than ideal so high baeyer strain, results in curvy weak C-C bonds with high P charecter
describe the conformation of cyclobutane
they pucker (decreasing bond angles) increasing baeyer strain, and make sure no bonds are fully eclipsed, reducing (but still high) pitzer strain, forming butterfly conformation
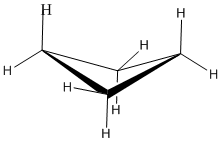
what is this conformation
butterfly conformation, found in cyclobutane
describe the conformation in cyclopentane
forms an envelope conformation, has some pitzer strain and some baeyer strain
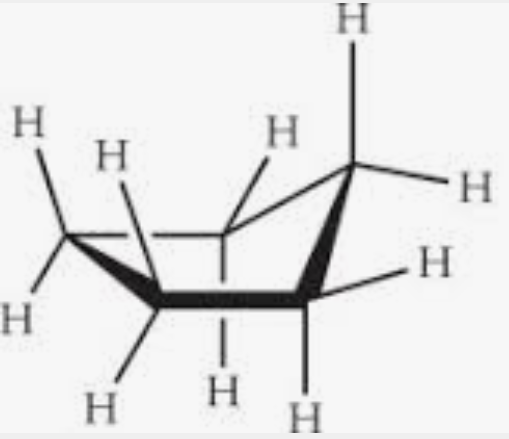
what is this conformation?
envelope conformation found in cyclopentane
describe conformation of cyclohexane
forms chair conformation, all bonds are staggered and essentially stain free, staggered conformation
what are the cyclohexane conformations with the highest energy to lowest
half chair, then boat, then twist boat, then chair
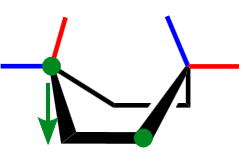
what cyclohexane conformation is this?
boat
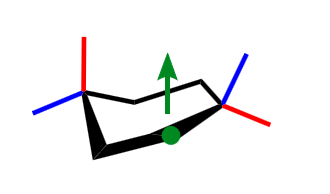
what cyclohexane conformation is this?
half chair
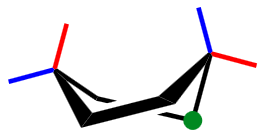
what cyclohexane conformation is this?
twist boat
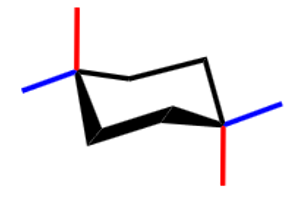
what cyclohexane conformation is this?
chair
what position is preferred for substituents on mono-substituted cyclohexane, why?
equatorial positions, this avoids 1,3-diaxial interactions
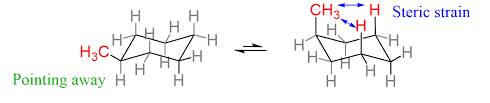
what is this showing
1,3 diaxial interactions on mono-substituted cyclohexanes
what is A-value showing
its a parameter to define size of substitutients, the E differance in kcal/mol bet
how do you find A-value
change in E (between highest and lowest conformation energy)
what is a locking group
a substituent on cyclohexane that locks it in one conformation, is a trimethyl group
when are di-substituted rings more stable?
when conformer has the largest group (measured by A value) in equatorial position
what are the 2 locking groups?
tB (tributyl methyl), and trans decalin
what is transdecalin
only conformer that exists is with central hydrogen in axial positions
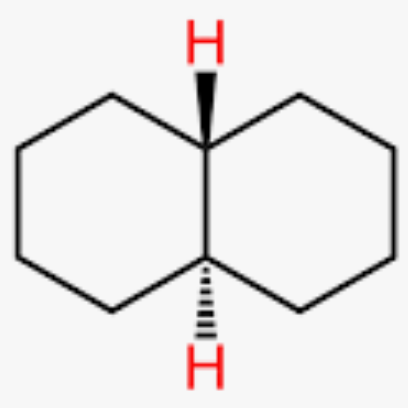
what is the J value for Hax,Hax?
10
what is the J value for Hax,Heq?
2-5
what is the J value for Heq,Hax?
2-5
what is the J value for Heq,Heq?
2-5
what is tetrahydropyran?
6 membered heterocyclic ring containing one oxygen, derived from pyran by saturation of double bond
what is the anomeric effect?
electronegative group at the anomric position, despite destabilising 1,3 diaxial interactions due to strong hyper-conjugation
what molecules is anomeric effect observed on?
molecules with OR, Cl, Br or F at anomeric position