Hydrocides, halides and phoshates
0.0(0)
Card Sorting
1/11
Earn XP
Description and Tags
Last updated 1:47 PM on 4/25/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
1
New cards
Kva er hydroxides?
* common, produced by wearhering and hydration of other minerals
* occur as fine-graind aggregates, usually mixed with other minerals
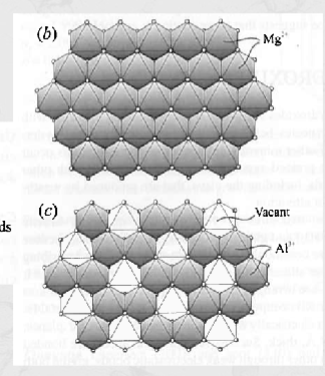
brucite Mg(OH)2 vs gibersite Al(OH)3 structure
* occur as fine-graind aggregates, usually mixed with other minerals
brucite Mg(OH)2 vs gibersite Al(OH)3 structure
2
New cards
Sei litt om brucite
* Mg(OH)2
* trigonal
* hexagonal mica-like plates, often aggregates
* H=2.5
* p=2.39 g/cm^3
* colorless in thin sections
* n=1.559-1.600 (low relief)
\
* trigonal
* hexagonal mica-like plates, often aggregates
* H=2.5
* p=2.39 g/cm^3
* colorless in thin sections
* n=1.559-1.600 (low relief)
\
3
New cards
Sei litt om Gibbsite
* Al(OH)
* monoclinic
* appears in soils, product of weathering of feldspars, major mineral in bauxite together with bömite and diaspore
* monoclinic
* appears in soils, product of weathering of feldspars, major mineral in bauxite together with bömite and diaspore
4
New cards
Sei litt om limonite
* iron hydoxide minerals
* it is a collectiv term used for mixtures of various Fe oxide and hydroxide minerals- most commonly:
* goethite - alfa-FeO(OH)
* lepifocrocite- y-FeO(OH)
* hematite (Fe2O3)
* intens weathering of Fe-bearing rocks may result in formation of lateritic soils rich in iron hydroxides- deposits of Fe ore
* yellowish brown or redish stain in weathering rocks is very-finegraind goethite, lepidocrocite hematite+ other minerals
* it is a collectiv term used for mixtures of various Fe oxide and hydroxide minerals- most commonly:
* goethite - alfa-FeO(OH)
* lepifocrocite- y-FeO(OH)
* hematite (Fe2O3)
* intens weathering of Fe-bearing rocks may result in formation of lateritic soils rich in iron hydroxides- deposits of Fe ore
* yellowish brown or redish stain in weathering rocks is very-finegraind goethite, lepidocrocite hematite+ other minerals
5
New cards
Sei litt om haldies
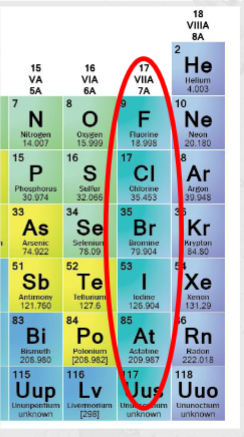
In halides, the anion is one of the halgeon elements F,Cl, Br and I. Their structure is made by ionic bonds of the halogens with other cations. Halides are highly soluble in H2O
6
New cards
Sei litt om Halite og sylvite
halite-NaCl, sylvite-KCl
* isometric symmetry
* crysrals are cubes with perfect (100) cleavage
* both minerals are isostructural with Na^+ or K^+ cations present in octahedrally coordinated sites surrounded by 6 Cl^- anions
* use: dietart purposes, manufacturing of industrial chemicals, metal processong, pharmaceutical industry, paper production. sylvite is used for as a source of potassium in fertilizer
* isometric symmetry
* crysrals are cubes with perfect (100) cleavage
* both minerals are isostructural with Na^+ or K^+ cations present in octahedrally coordinated sites surrounded by 6 Cl^- anions
* use: dietart purposes, manufacturing of industrial chemicals, metal processong, pharmaceutical industry, paper production. sylvite is used for as a source of potassium in fertilizer
7
New cards
Sei litt om Fluorite
CaF2
* isometric symmetry
* colourless, blue, purple, green, yellowish…colours
* crystals are cubes with perfect (111) cleavage
* in fluorite,Ca^2+ cations are present in 8-fold coordinated sites (surrounded by 8F^- anions)
* use: principal source of F for chemical processes. it is also used as melting agent (flux) in steel production
* isometric symmetry
* colourless, blue, purple, green, yellowish…colours
* crystals are cubes with perfect (111) cleavage
* in fluorite,Ca^2+ cations are present in 8-fold coordinated sites (surrounded by 8F^- anions)
* use: principal source of F for chemical processes. it is also used as melting agent (flux) in steel production
8
New cards
Sei litt om Gypsum
(CaSO4x2H2O)- monoclinic symmetry
* with, pale gray, brown, yellow…
* crystals are mostly tabular parallel to (010)
* common twinning along (100) plane
* large euhedral crystals are called selenite
* granular massive gypsum is called alabaster
* with, pale gray, brown, yellow…
* crystals are mostly tabular parallel to (010)
* common twinning along (100) plane
* large euhedral crystals are called selenite
* granular massive gypsum is called alabaster
9
New cards
Sei litt om anhydrite
CaSO4
* orthorombic symmetry
* colourless, with, gray, bluish..
* crystals are blocky or tabular, often just massiv
* gypsum: H=2
* anhydrite: H=3-3.5
* use: anhydrite is mixed with portland cement to control the rate of curing (hardening). when prepared in lab, CaSO4 is used as carrier for antiviotics and in some other medical applications
* orthorombic symmetry
* colourless, with, gray, bluish..
* crystals are blocky or tabular, often just massiv
* gypsum: H=2
* anhydrite: H=3-3.5
* use: anhydrite is mixed with portland cement to control the rate of curing (hardening). when prepared in lab, CaSO4 is used as carrier for antiviotics and in some other medical applications
10
New cards
Sei litt om barite
BaSO4
* orthorombic symmetry
* withe, pale yellow, gray, pale green…
* crystals are tabulare parallel to (001), often intergrown to form rosettes or just granulart masses
* H=2.5-3.5
* p=4.5 g/cm^3
* use: most barite is mined as industria, mineral in order to use its high specific gravity. it is used in the petroleum industry as a componet of drillling mud in oil and gas wells. glassmaking, ceramics, high-density filler in plastcs and rubber
* orthorombic symmetry
* withe, pale yellow, gray, pale green…
* crystals are tabulare parallel to (001), often intergrown to form rosettes or just granulart masses
* H=2.5-3.5
* p=4.5 g/cm^3
* use: most barite is mined as industria, mineral in order to use its high specific gravity. it is used in the petroleum industry as a componet of drillling mud in oil and gas wells. glassmaking, ceramics, high-density filler in plastcs and rubber
11
New cards
Sei litt om apatite
Ca5(PO4)3(OH,F,Cl)
* hexagonal symmetry
* grayish blue-green, geen, yellow..
* colourless in thin section
* crystals are hexagonal prismatic
* n=1.629-1.666 (medium relief)
* d= 0.001-0.007 (gray interf. col.)
* OH
* hexagonal symmetry
* grayish blue-green, geen, yellow..
* colourless in thin section
* crystals are hexagonal prismatic
* n=1.629-1.666 (medium relief)
* d= 0.001-0.007 (gray interf. col.)
* OH
12
New cards
Sei litt om Monazite og Xenotime
monazite-(Ce,La,Th)PO4
* monoclinic symmetry
* yellow, reddish yellow or brown
* yellow or colourless in thin sections
* tabular crystals
* n= 1.772-1.860 (high relief)
* d=0.045-0.075 (3rd-4th order)
Xenotime-YPO4
* tetragonal symmetry
* elongate tetragonal prisms
Use in sicence: both concentrate U and are being used for high-temprature (U-Pb) geochronology
\
* monoclinic symmetry
* yellow, reddish yellow or brown
* yellow or colourless in thin sections
* tabular crystals
* n= 1.772-1.860 (high relief)
* d=0.045-0.075 (3rd-4th order)
Xenotime-YPO4
* tetragonal symmetry
* elongate tetragonal prisms
Use in sicence: both concentrate U and are being used for high-temprature (U-Pb) geochronology
\