bio230 2nd half
1/261
Earn XP
Description and Tags
these are basically my class notes
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
262 Terms
Lec 1-3: How are cells and tissues organized spatially?
1: Membrane Trafficking
Cell Polarization
Some Cells
Polar=different at ends
Different functions based on cell regions (signal, separate, etc)
Ex. Epithelial cell basolateral domain vs apical domain
Membrane trafficking
Moving stuff to different membranes, determines where proteins end up
Eg. Move proteins to different polar domains
2 ways:
Exocytosis to target domain
Exocytosis to any domain then selective endocytosis/ recycling to target domain
Sorting stations
Organizes proteins during membrane trafficking
2 main sorting stations:
Trans golgi netwrok
Endosome
Secretion pathways (3)
Constitutive
Regulated (signal mediated)
Lysosomal (signal mediated)
Constitutive secretion
Default pathway
Specific signals not required
Trans golgi network —> vesicle —> membrane
TM proteins present
Adds phospholipid
Regulated secretion
Release material in response to a signal
Fully formed vesicles that dont fuse to a membrane unless signal is present
TM protein also present
Eg. Mast cell releases stored histamine in allergic reactions
Regulation can give a boost of phospholipid to PM, eg after loss of membrane in phagocytosis, wound, or cytokinesis
How can Concentrated cargo occur?
Clathrin coated vesicle buds off cargo vesicle to shrink it and goes back to golgi with just fluid
Both constitutive and regulated can release conc. cargo
Endocytosed proteins (membrane trafficking)
membrane trafficking option 2 : exocytosis all proteins, then selective endocytosis of some of those proteins to another domain (can add to polarity)
3 options:
Recycle to same domain: protein binds to receptor →early lysosome → recycled BACK to og/same domain
Transcytosis: moved to other side/domain of PM; protein binds to receptor →early endosome→transport vesicle→recycling endosome →transport vesicle→other membrane
Degradation : in the lysosome
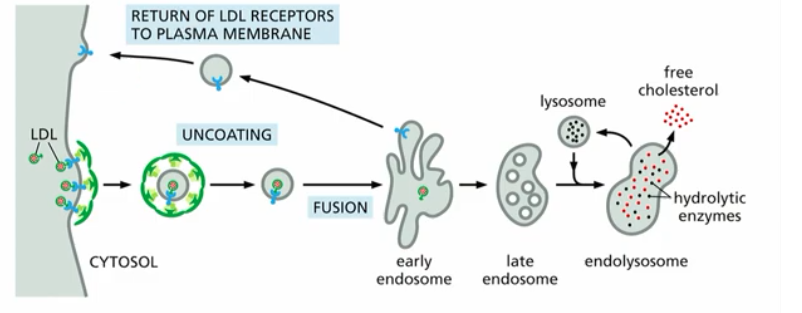
example: cholesterol uptake
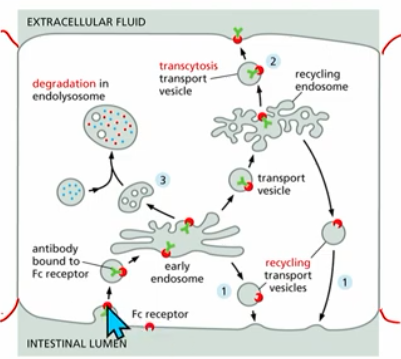
Cholesterol Uptake (Ex. of Endocytosis in Membrane Trafficking)
process overview:
Low Density Lipoprotein (LDL) binds to cholesterol → LDL-C bound to PM receptor →clathrin coat vesicle forms (clathrin coat selects cargo, gives curvature to vesicle, promotes vesicle budding)→unncoating→fusion (to early endosome)
then, s basic principles
recycling of receptor
transcytosis of free cholesterol
degradation of cholestorol in late endosome, endolysosome, lysosome,
3 types of membrane changes (during vesicle tracking)
(endocytosis) vesicles forming from donor membrane into cytoplasm
*(outside of cell or lumen of organelle)
eg. COPI/COPII vesicles going to/from ER & Golgi(exocytosis) vesicle fusion: vesicle merges with target membrane
*(outside of cell or lumen of organelle)
eg. SNARE proteins (the pullers). Both t-SNAREs & v-SNAREs; opposite membranes to fuse.Vesicle forms from donor membrane away from cytoplasm
*(eg virus leaves to outside of cell and may take a bit of PM with it; may carry RNA to communicate with other cells)
* ESCRT proteins form vesicles using machinery in cytoplasm (see image).
3 is the only one leaving the cell with part of the PM (net loss of PM in cell)
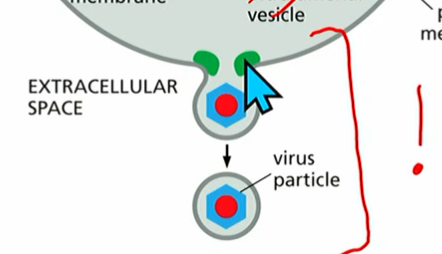
ESCRT proteins (cont.)
From membrane change #3 (the new, virus one that takes PM with it using machinery in the cytoplasm )
many ESCRT proteins (0-3) form vesicles, passing molecules down :
ESCRT-0 activated by a) PI(3)P and b) multiubiquitinated TM viral protein on PM
passes off a chain of ESCRT-1-3.
ESCRT-3 builds up around it and will form the vesicle
Lipid Changes During Membrane Trafficking
phospoinositides (PIPs) are found at different subcellular locations
different domains/compartments contain diff. lipids
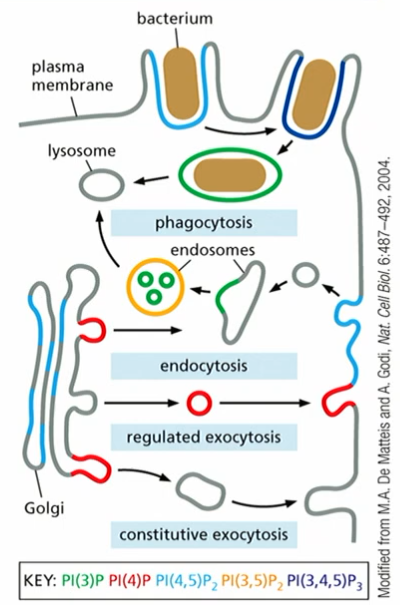
PIPs
phospoinositides
part of machinery in membrane change #3
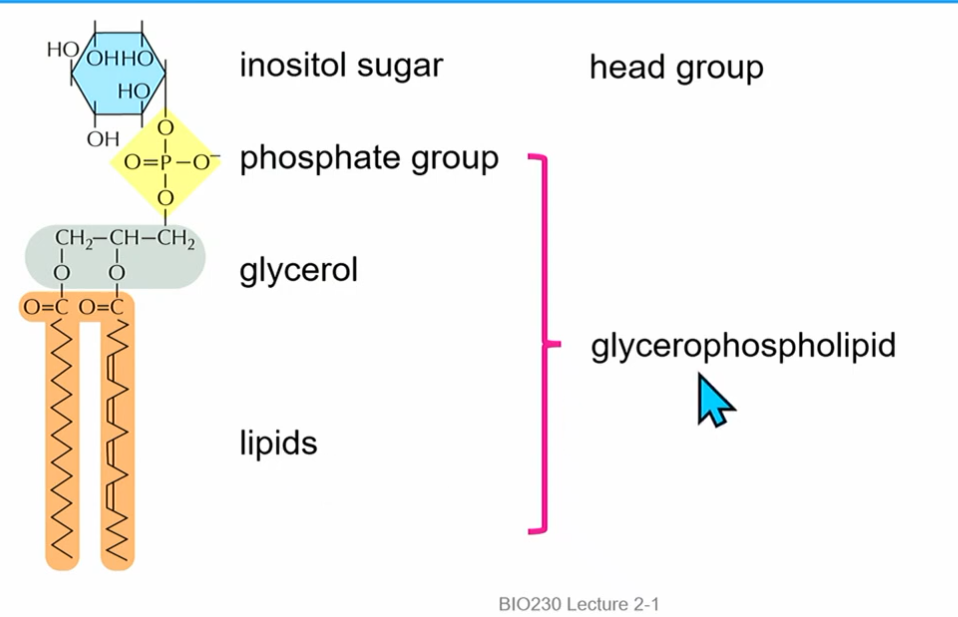
inosital sugar(**6Cs, phosphate attached to C1, count CCW; possible additional phosphorylation sites), structural phosphate (alw there), glycerol, phosphatidylinositol (PI)
2 phosphorylations on the inosital sugar at C3 and C4 : PI(3,4)P₂
interconverted by kinases and phosphatases (not every kinase/phosphatase exists)
can use map
different proteins bind to different PIPs which directs membrane trafficking
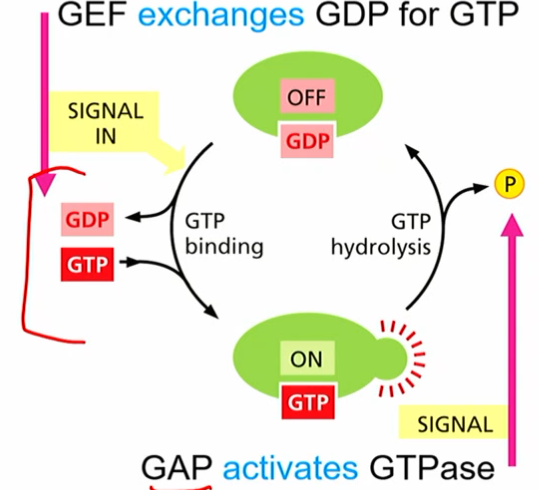
Rab-GTP
molecular switches found on vesicle or target membranes
help with vesicle formation, “matching” so correct vesicle gets brought where it needs to go
together with PIP, can give membranes diff identities
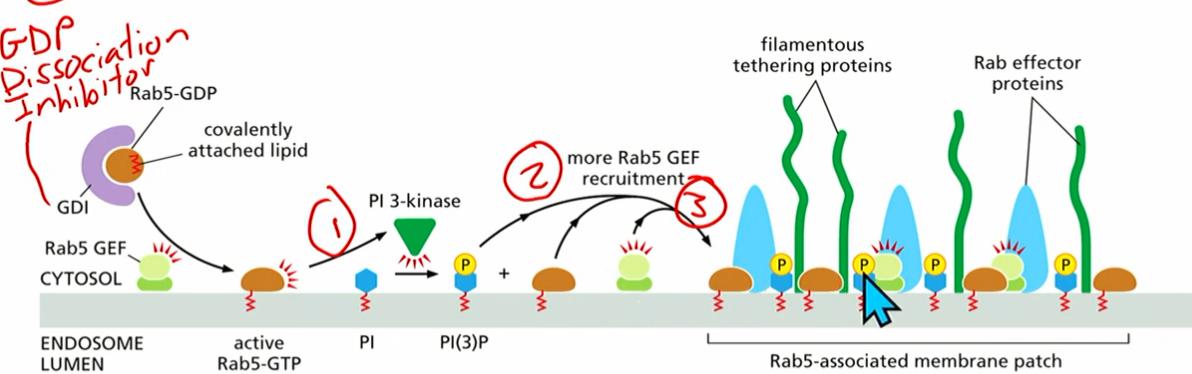
Rab 5
rab 5 gtp recruits PI3 kinase
pi(3)p can recruit rab5-gef
make more active rab5-gtp
pi3p and rab5gtp activate tethering proteins
*GDI (gdp dissociation inhibitor) bound to inactive rab5gdp
2: Cytoskeletal Networks
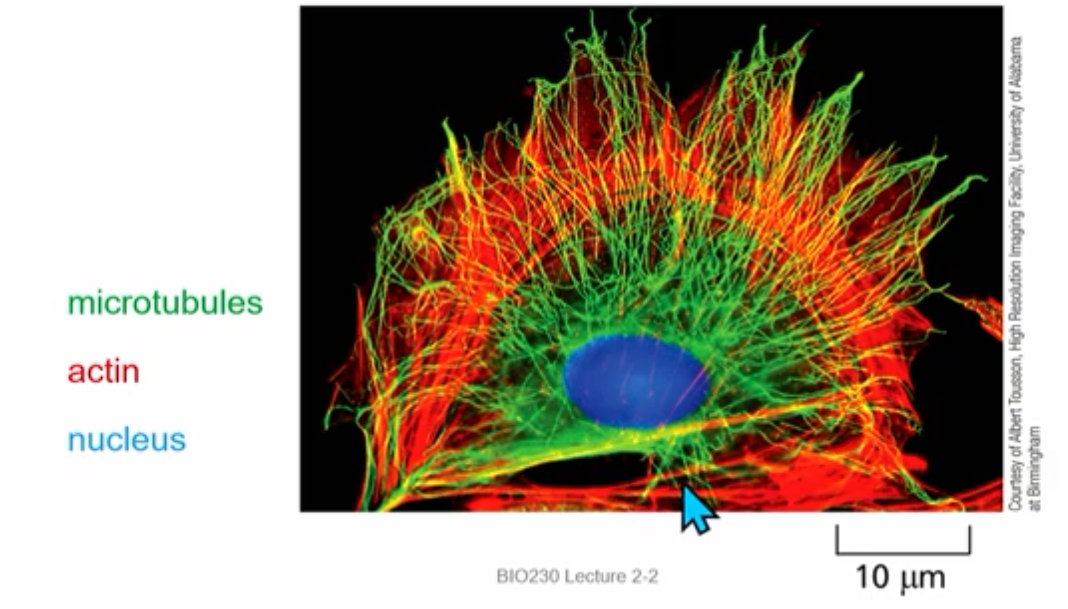
Polar Cytoskeleton Organization
Polar…
microtubules: transport vesicles and proteins to ends of cell
actin: define cell shape and behaviour
intermediate filament: contribute to cell polarity
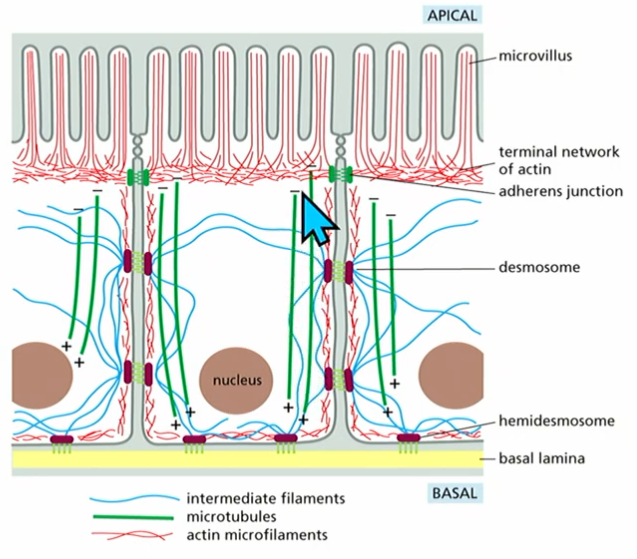
Polar microtubules
transport vesicles and proteins to ends of cell
eg. + end, - end
Polar actin
define cell shape and behaviour
eg. in microvilli at the ends
Polar intermediate filament
also contribute to cell polarity (more detail not req.)
Cytoskeleton dynamic rearrangments
Growth and Shrinkage
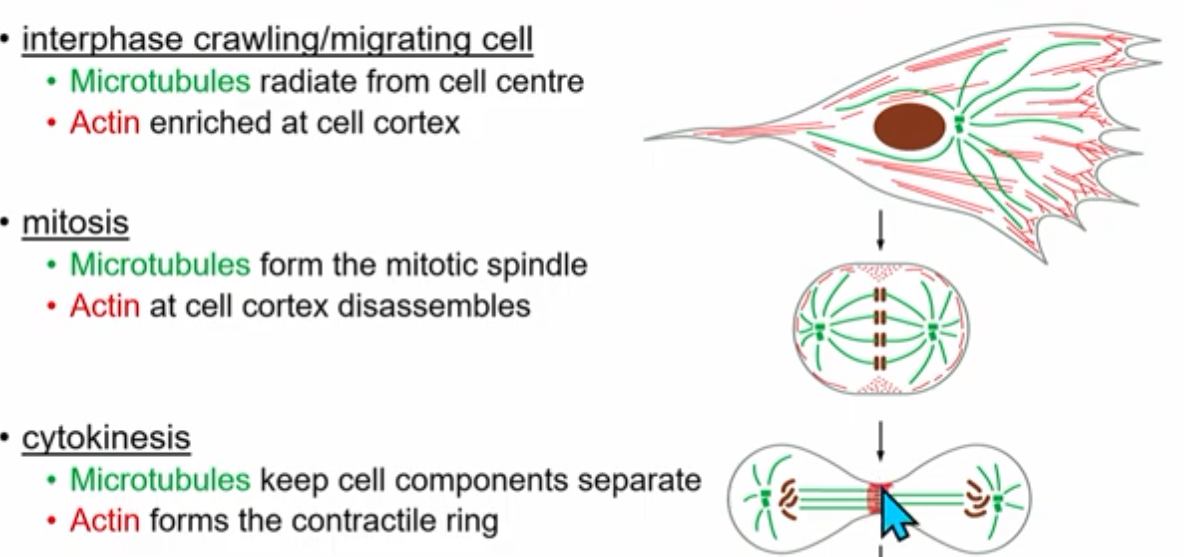
microtubules
dimers made of monomeric proteins alpha tubulin (- end) and beta tubulin (+ end)
*+,- ends are convention, not chargetubulins bind and hydrolyze GTP
tubulin heterodimers assemble head to tail to make polarized protofilaments (alternating top to bottom)
13 parallel (crossection = all beta or all alpha) protofilaments form a hollow microtubule
after being protofilament for a while beta tubulin will cut GTP to GDP
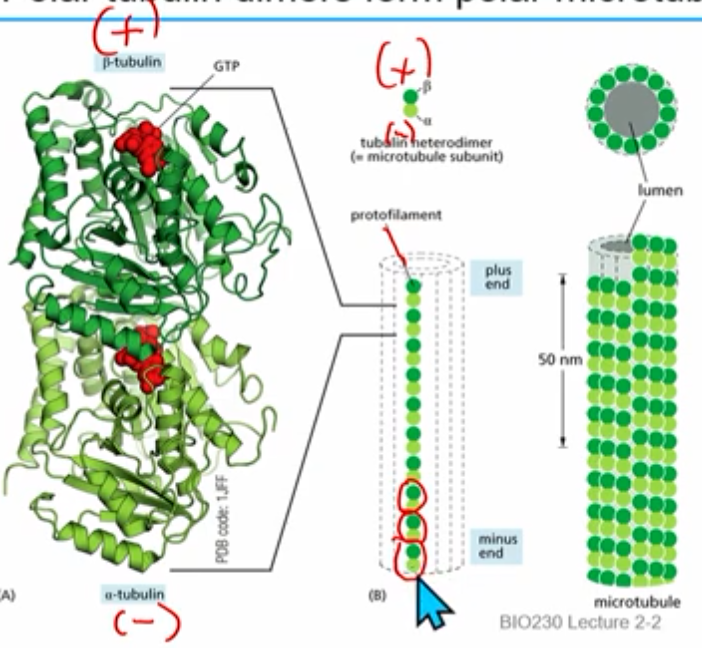
T form heterodimers vs D form heterodimers
t form: alpha and beta tubulin bound to GTP
*t form ends usually means growth/addition
d form: alpha GTP but beta tubulin bound to GDP
*d form ends usually means shrinkage/subtraction
GTP cap (dynamic instability)
everything starts as T form; over time cut to D form- the freshest added (the cap) is still T form
when microtubule (beta tubulin, +) is in T form and in growing/addition and the cap is not yet hydrolyzed to GDP (→ D form)
dynamic, time sensitive
after a while GTP → GDP
if ends in D form, rapid shrinkage
gamma tubulin
what about other end of microtubule (shrinkage?)?
gamma tubulin nucleates alpha ( - ) end and protects from depolymerization
growth radiates from gamma tubulin end (- end)
remains even if d form shrinkage
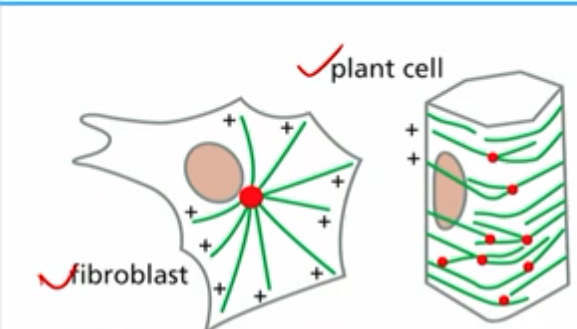
patterns of nucleation create microtubule patterns
how do patterns of nucleation create microtubule patterns?
fibroblast:
microtubules radiate away from centrosome (2 centrioles), surrounded by pericentriolar material (pcm) which gamma tubulin ring complexes are found
plant cell:
gamma tubulin is found on other microtubules
“branching” or seeding from another microtubule (augmin connector)
MAPs
microtubule associated proteins
kinesins “walk” towards the plus end generally
dyneins move towards minus end generally
both can hold onto vesicles/organelles with other domains and use ATP hydrolysis
Tilapia using MAPs motor proteins
microtubule motor proteins can change fish color
Kinesins walk to + end; dyneins walk to - end
In dark fish they both carry vesicles with pigment and compete with each other; even distribution of pigment.
In light fish kinesins are inhibited, pigment is no longer moved outwards and tightly aggregates in the cell centre; light pht is observed.
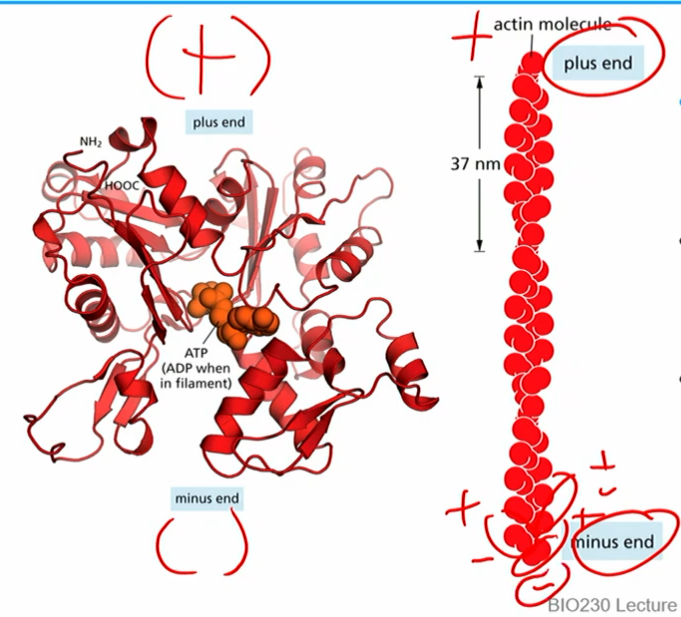
polar actin filaments
made of monomers that are asymmetric and thus “polar”
can bind and hydrolyze ATP
by convention (not charge), - end bottom, + end top
2 monomer strands assemble (twizzler style) into polarized actin filaments
plus end literally ends with a +monomer and vice versa
T form monomers (actin ATP) and D form monomers (actin ADP)
*same basic concept as microtubule time sensitive GTP→GDP bound
Treadmilling
actin filaments soluble subunits in tform
polymers are a mixture of tform and dform(old)
at the right conc. of actin: some addition of actin at + end but not at the minus end (concurrent loss at - end, gain at + end)
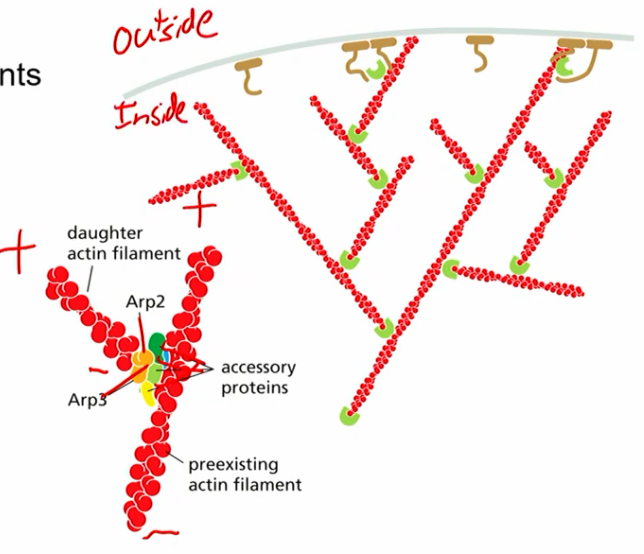
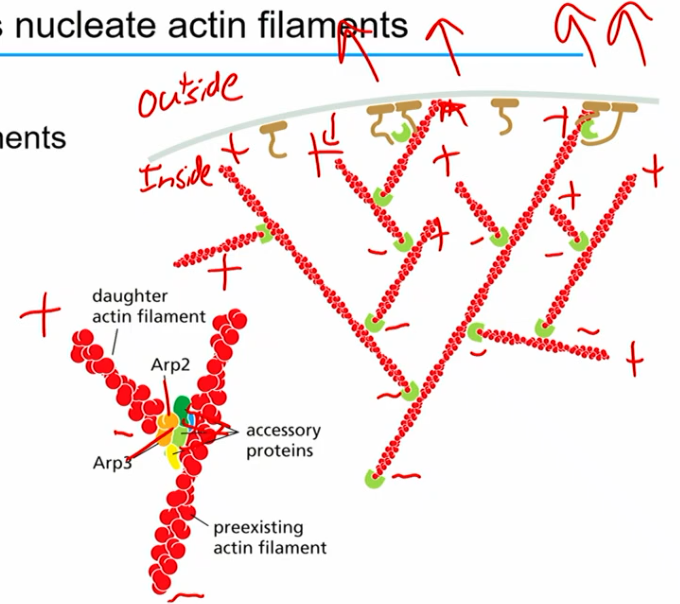
ARP2/3 complex
actin related proteins
helps nucleate actin filaments (stabilize - end of actin and protect from depolymerization)
equivalent of gamma tubulin in microtubule?
uniquely nucleates actin filaments on pre-existing filaments (branched, seeding thing)
creates polar network of actin in cell (generally + towards membrane, - inside)
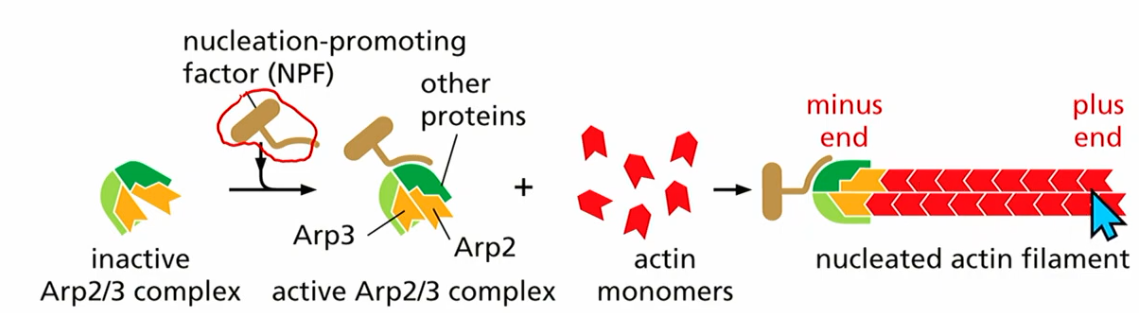
NPF
nucleation promoting factor
NPF binds to inactive Arp2/3 complex
t form actin assembles on other plus end
Ways actin can move (4)
elongate
shrink
treadmill
stabilize with arp2/3
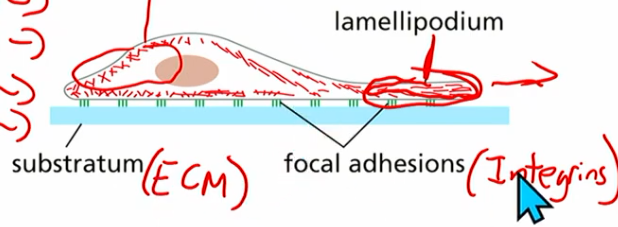
Cell crawling (how can a cell move with actin?)
uses polar actin network
actin getting added to leading edge (membrane region of cell)
new branches from arp2/3 poking the membrane forward
actin treadmilling (poke forward while loss on other end)
basically: poke poke poke, other side contractile & getting dragged along
cofilin
chopping protein
cuts actin filament; exposed d form actin gets degraded quickly
release from arp2/3 to help deassemble actin
found in network treadmilling (where + end poking)
Integrins
TM heterodimers (alpha, beta subunits)
anchor actin to extracellular matrix (ECM) proteins
indirectly interact with actin
provide adhesion necessary for cell migration
other proteins help w this too
myosin
motor proteins; motor domains use atp for motor domains
works with actin to generate force (cell migration, muscle contraction)
Rho family small GTPases
influence actin organization → cell shape, polarity and behaviour
act as molecular switches
RhoGAP and RhoGEF, GTP binding and hydrolysis (similar concept to RanGTP in nuclear transport)
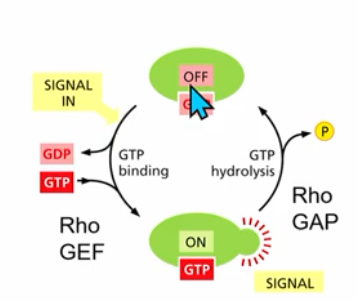
Rho family GTPases members? (3)
Family members: Rho, Rac, Cdc42 proteins
overactivation of any members leads to varying actin organization patterns (next slides)
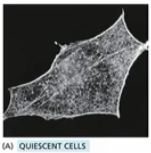
Quiescent cells
normal Rho family GTPase activation (normal actin behaviour, cell movement), healthy balance b/w members
cell in interphase
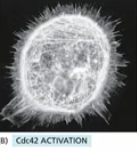
Cdc42 overactivation
Cdc42 overactivation (lots of Cdc42 gef) → spikes
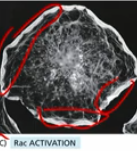
Rac overactivation
Leading edge around the entire cell
not normal
Rho overactivation
myosin contraction throughout the cell (as opposed to only the dragged behind end)

Rac GTP activation
responsible for leading edge “poking” behaviour
in a normal cell Rac GTP activation occur mainly at front of cell
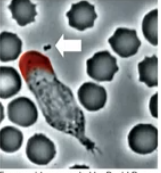
Rho GTP activation
responsible for contractile behaviour
in a normal cell, Rho GTP activation occurs at “back” of the cell
(eg. this neutrophil chase)
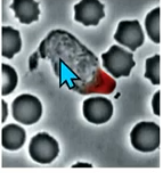
chemoattractant
bacterial waste(?) that notifies a neutrophil (bacteria eater cell)
that activates receptor for Rac GTP to form leading edge
cell polarity in fertilized egg/zygote
sperm (posterior) enters egg ; symmetry breaking occurs
cytoskeleton changes to polar
bigger theme of blob →distinct phenotype
3: Cell Adhesion
cell cohesion
types of tissue work tgt (eg.?)
essential for multicellular organisms
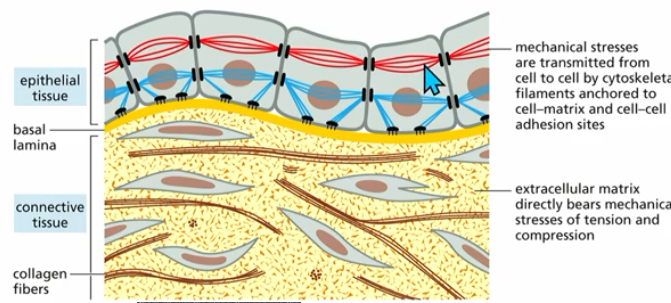
epithelial cells
line surfaces, cavities, and organs
protective (skin)
absorption (digestive tract)
define (organs)
polarized: apical, basal
epithelial structure
Apical (top)
basal (bottom; facing basal lamina)
require junctional complexes

epithelial junctions (6)
apical
tight: seal gap bw ep cells, block, keep stuff on right side, form sealing strands
cell-cell anchoring junctions:adherens: deeper into plane, 3d adhesion, form adhesion belt, anchors actin bundles of two cells O-O
desmosome: connects int. filaments, anchors int. fil of two cells
gap: lets small soluble (water) thru bw cells
actin linked cell matrix ALCM: sticking cell to ECM via actin + ECM proteins
hemidesmosome : anchors int fil to ECM
*these last two are next to each other in the bottom
basal
epithelial junction (category)
apical
tight junction
tight
cell-cell anchoring junctions (2)
adherens
desmosome
channel forming junction
gap
cell matrix anchoring junctions (2)
ALCM
hemidesmosome
basal
how do cell-cell junctions work?
mediated by cadherin family members
use TM adhesion proteins (integrins) to stick two cells
actin or int. fil.s
cadherin family members
@ adherens junctions
TM proteins expressed by both cells, stick to each other outside of cell
like velcro
homophilic intc.s (only the same types of cadherins stick to each other)
req. Ca2+ to stick to ea other (keeps it rigid)
homophilic cadherin interactions
allow cells to sort into groups (like the hydrophobic effect)
cells with E-cahderins; cells with N-cadherins
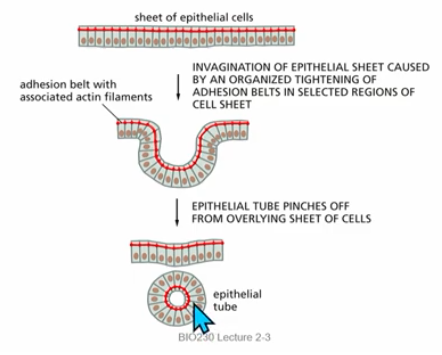
adhesion belts
formed by adherens junctions (which anchor actin bundles of 2 cells)
can mediate morphogenesis, embryogenesis
contraction can form tube structures (eg formation of spinal chord)
apical domain ep cell
faces surface, cavity, organ
basal domain
faces inside of the body
basolateral domain
basal and lateral domains
tight junction
physically defines domains (apical, basal, etc)
limits diffusion from one cell to the next
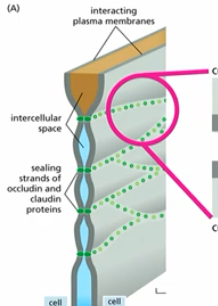
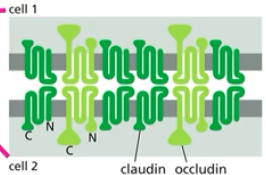
occludins & claudins
monomers of tight junction (many rows make up a )
TM proteins that form homophilic interactions with their extacellular domains to directly link adjacent cells
tight junctions & glucose conc.
glucose conc. (low outside ep cell, high in centre of ep cell) maintained by transporters being on the right side (apical vs basolateral) by tight junctions
how?
glucose found in lumen of gut - food filled cavity lined with epithelial cells
apical side contains:
sodium driven (active) glucose transporters (kept on apical side by tight junctions) →high conc. glucose pumped in cell
basolateral side contains:
passive glucose transporter proteins (kept on that side by tight junctions)→glucose leaves cell (eg. thru bottom; basal lamina to blood)
glucose has to go through cell; cant passively diffuse to blood/connective tissue
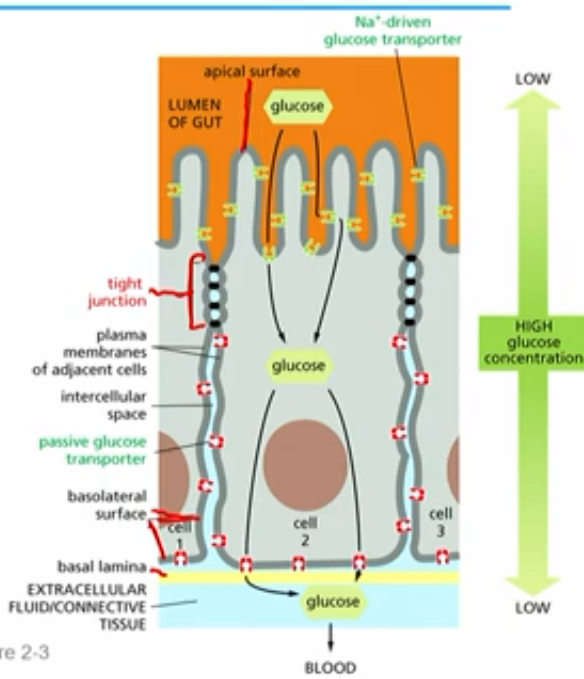
sodium driven (active) glucose transporters
moves glucose from apical into ep cell
creates low to high conc grandient
passive glucose transporter proteins
allows glucose to diffuse from basolateral side to basal lamina/blood
(to adjacent cells???)
cell-matrix junctions
play roles in both epithelial and connective tissue
what order/cues of junctions are made in ep cell?
adherens junctions form first:
help cells stick together
provide polarity cues to define apical domain from basolateral domain
adherens junctions give polarity signals that activate PAR, Crumbs, Scribble complexes
tight junctions
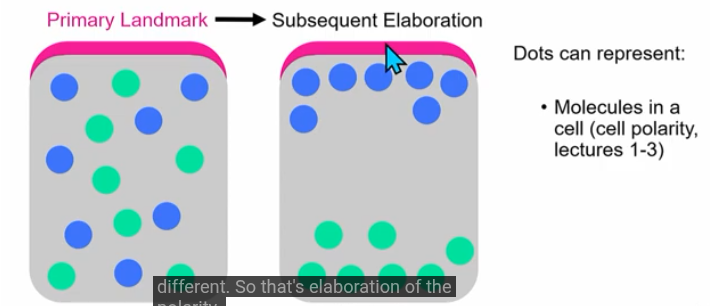
landmark
can be a structure, protein, signal or process
initial landmark can generate subsequent patterns/elaboration
eg. adherens junctions is landmark; leads to cell polarity
Lec 4-6: How do multicellular organisms develop?
4: Tissue Morphogenesis
(3) key concepts in multicellular development
1) cell proliferation
2) cell differentiation
3) cell morphogenesis
cell proliferation
increase in cell numbers
cell division
(eg cleavage of fertilized egg to the blastula)
cell differentiation
change in cell fate (function, location) via cell signaling and differential genome expression
(eg. gastrulation of blastula to gastrula)
cell morphogenesis
change in cell shape, interactions and/or locations
(eg. gastrulation of blastula to gastrula)
embryogenesis
initial stages after fertilisation of the egg
model system for multicellular dev:
busy time for multicellular development + short reproducible timeline → great to study these processes
does multicellular development occur in adults?
yes, particularly in stem cells
ongoing (eg skin/ep cells) and new (eg. pregnancy) developmental changes
embryogenesis steps
1) cleavage (many cell divisions) of fertilized egg
2) gastrulation of blastula to create gastrula
fertilized egg → (cleavage) →blastula →(gastrulation)→ gastrula
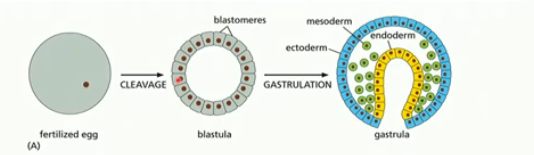
blastula
sphere of cells, hollow/ filled with fluid
created by cleavage/many cell divisions
fairly undifferentiated (can become any cell)
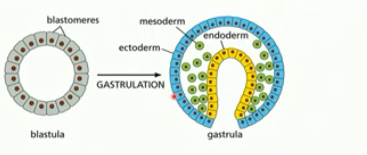
gastrulation
change from ball of cells to embryo with a gut and 3 germ layers (eg. ectoderm, mesoderm, endoderm)
formation of the gut tube (yellow) eventually forms the digestive tract
blastula folds in on itself
cell types begin to emerge
partial differentiation
morphogenesis
the generation of shape
(3) key concepts of morphogenesis
cell internalization
elongation
fine repositioning of cells
(how does gastrula esque blob acc become an animal looking thing)
cell internalization
delamination, ingression, involution, invagination
3 germ layers
each layer becomes different kinds of cell tissues in the adult body
ectoderm, mesoderm, endoderm
ectoderm
becomes the epidermis & nervous system
mesoderm
becomes the muscles, connective tissue, bones, blood, kidneys, etc
endoderm
becomes gut, lungs, pancreas, liver, etc
stupid TCP analogy of gastrulation/3 layers
blastula is kinda like if you have the younger group of students that could become any specialty, then once gastrulated the older kids pick a path and still could do a variety of things, but cant go back and do anything. like endoderm cells can become pancreas, gut, lungs but they cant go back and become NS or skin. its like choosing medicine pathway and you can be a nurse, researcher, surgeon but not a graphic deisgner or some shit idk. making me think abt the academy and its reach. how many things does it do? what does it train its kids to become? is it monopolizing anything? roots deep in the cities etc. anyway. back to lec
(2) process to form mesoderm
ingression and delamination
ingression
process to form mesoderm
individiual cells detach from the outer cell layer and migrate (green, cells lost adhesions)
aka epithelial-to-mesenchymal transition
delamination
process to form mesoderm
cells forming a new layer (mesoderm)
epithelial-to-mesenchymal transition
ingression
cell changed from epithelial cell (tighly adhered to neighbors) to crawling type cell (mesenchymal cell)
tightly controlled; potential for malignant cell growth
(2) processes to make endoderm
invagination
involution
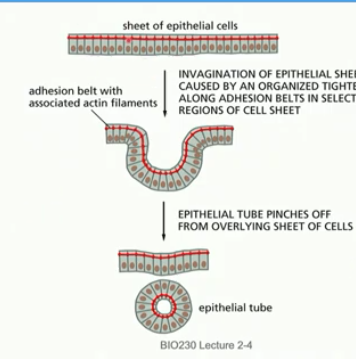
invagination
form endoderm
sheet of tight cells curls in to form pouch/cavity
elongation of mictrotubules in sheet of ep cells → invagination by adhesion belt tightening in select areas
*req actin, cell adhesion
involution
curling in of the cells
gut tube
one end develops to become the mouth, the other end develops to become the anus
forms from endoderm
vertebrate neural tube
forms similar to gut tube (invagination/involution)
ectoderm → neural plate cells → neural crest plate →neural tube
relies on dev signalling, diff. gene expression (eg. of cadherin)
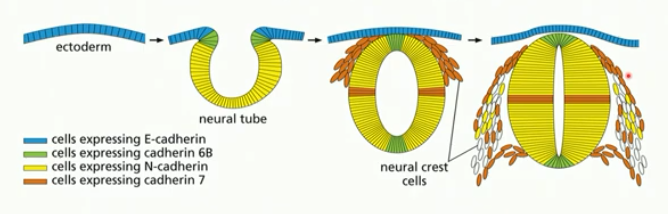
elongation
congergent extension, mass cell migration, asymmetric growth
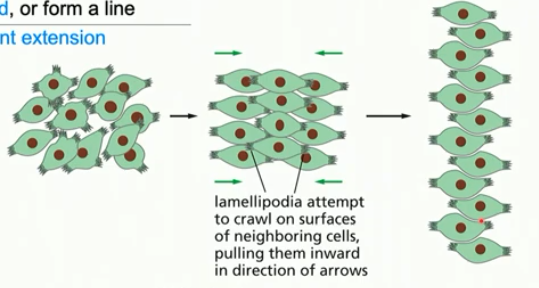
convergent extension
involves cell movement
cells come together (converge) and line up (extend as a tissue)
same number of cells throughout; organiszation differs
after gastrulation