Chem unti 1 quiz
1/16
Earn XP
Description and Tags
Identifying Physical & Chemical Properties Identifying Physical & Chemical Changes States of Matter & Phase change names
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Physical Properties
Characteristics that can be observed w/ out altering the identity of the substance
density, color, melting point, state of matter
Chemical Properties
Characteristics of a substance that cannot be observed without altering the substance
flammability
Physical Change
Does not alter the identity of the substance
phase change
Chemical Change
Alters the identity of the substance
burning
Indications of Chemical change
Transfer of energy, change in color, production of a gas, formation of a precipitate, change in pH
Precipitate
A solid that forms and settles out of a liquid mixture
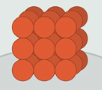
Solid
Definite shape & volume, not easily compressed, strong forces of attraction between particles

Liquid
Indefinite shape, definite volume, not easily compressed, weak forces of attraction between particles

Gas
Indefinite shape, indefinite volume, easily compressed, no attraction between particles
Sublimation
Solid to Gas
Deposition
Gas to Solid
Melting
Solid to Liquid
Freezing
Liquid to Solid
Vaporization
Liquid to Gas
Condensation
Gas to Liquid
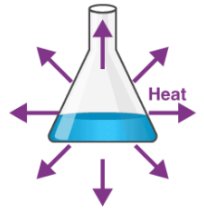
Exothermic
Reaction that releases energy into the environment (heat increase)
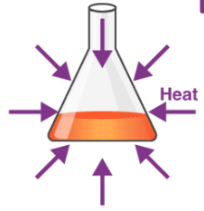
Endothermic
Reaction that absorbs energy from the environment (heat decrease)