IB ESS Unit 6
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
The Atmosphere and its Functions
The layers of gases surrounding the Earth. Its atmosphere is composed of ~78% nitrogen, ~21% oxygen, and one percent other gases.
It provides a shield from meteorites.
It protects us from harmful radiation from the sun.
It moderates and stabilizes our climate including temperature.
It is from where we obtain the oxygen we breathe and from where plants acquire the carbon dioxide they require for photosynthesis.
Layers of the atmosphere
Closest to Earth:
Troposphere: Where all the weather patterns happen, temperatures decrease
Stratosphere: Ozone layer takes place here, temperatures increase here
Mesosphere: temperatures decrease with altitude
Thermosphere: absorbs ultraviolet radiations, so temperatures increase
Exosphere: gradually translates to outer space, the last layer.
Furthest away from Earth
Greenhouse effect
Without GHG’s the heat is radiated back into space, resulting in average global temperatures of -18 C
With GHG’s, long wave radiation is absorbed by gases, resulting in warming of the atmosphere to average temperatures of 15 C. This is the natural greenhouse effect.
We see that increasing the GHG’s in atmosphere results in an unnatural enhanced greenhouse effect, warming the planet.
Albedo Effect
the ability of surfaces to reflect sunlight. linked to how white the surface is. clouds reflect and absorb a lot of solar energy and also trap heat below them depending on how thick and high they are
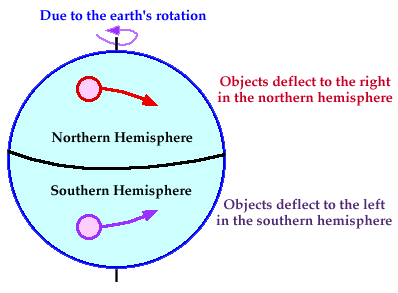
Air Circulation and the Corilis effect
Rising air: warm and moist, low-density, low-pressure area
Falling air: cool and dry, high density and high-pressure area
Corilis Effect → Because of the spin of the earth, objects – air currents, water and current deflect makes storm swirl clockwise in the southern hemisphere and counterclockwise in the Northern Hemisphere
Ozone & its formation cycle
Ozone is a replenishable, colourless, unstable toxic gas with powerful oxidising properties. The ozone layer is found in the stratosphere and shields us from much of the sun's ultraviolet radiation.
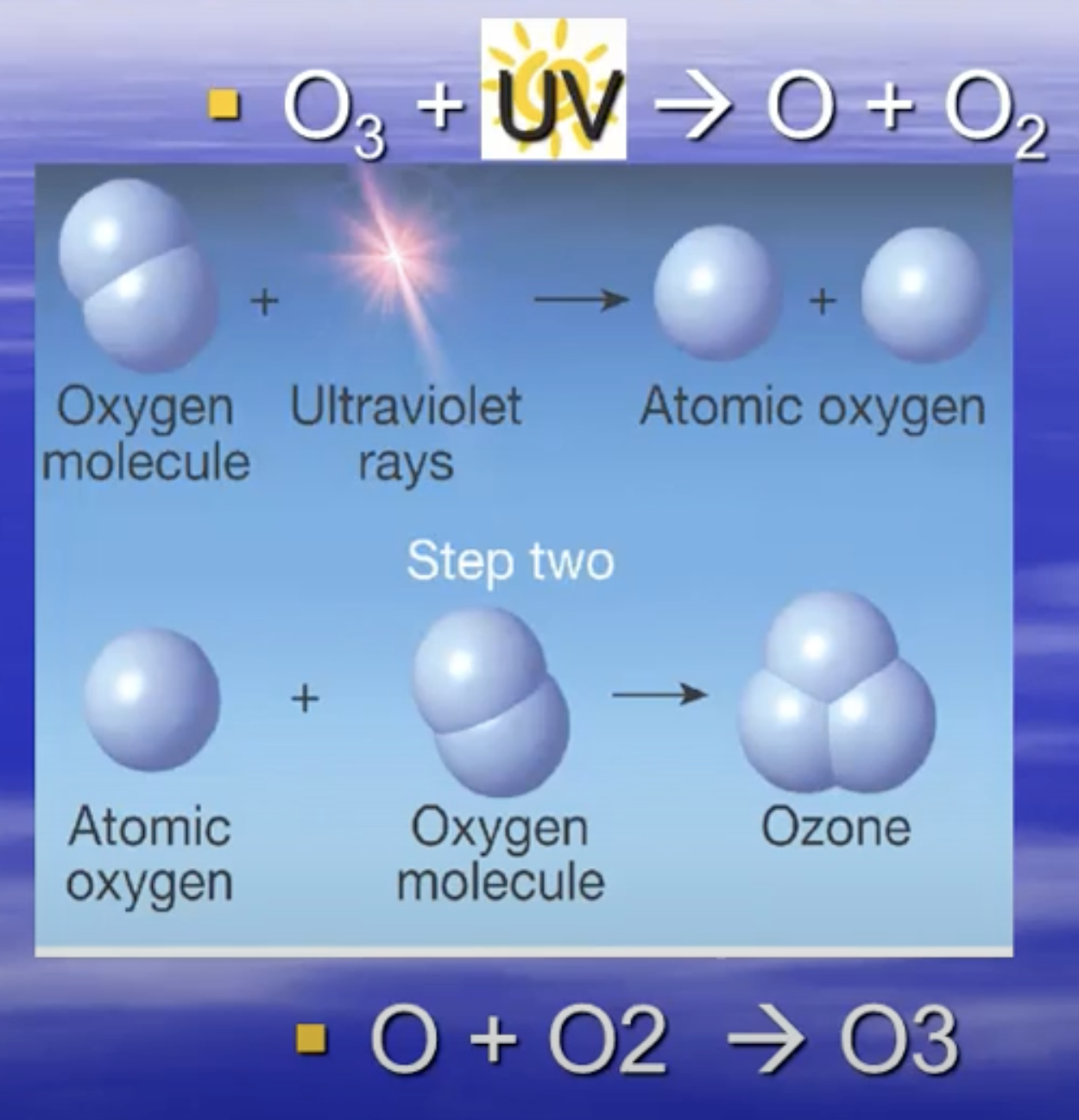
Ozone Cycle → When UV rays hit ozone (O3), the molecule separates into an ozone radical (O) and Oxygen (O2). The radical ozone continues to travel around until it hits Oxygen again, combining back into Ozone
Chlorofluorocarbon Compounds (CFCs) - uses, benefits & disadvantages
CFCs → nontoxic, nonflammable chemicals containing atoms of carbon, chlorine, and fluorine.
Used as coolants (refrigerators), propellants (hairsprays and deodorants), fumigants (bug spray), etc.
Benefits:
chemically stable
nontoxic
odourless
nonflammable
noncorrosive
Disadvantages:
discovered to be lowering the concentration of stratospheric ozone (more UV getting through)
UV radiation splits chlorine atoms from CFCs which combines with radical ozones, depleting the concentration of ozone
Impacts of UV rays and management strategies
Impacts:
Worse sunburns
More eye cataracts and skin cancers
Immune system suppression
Damage to producers (like plants and phytoplankton) due to sensitivity, affecting entire ecosystems.
Reduced yield for sensitive crops
Management Strategies:
Replace
use eco-friendly spray and coolant products instead of CFCs - this is hard without bans/fines
education and campaigning (EPA Clean Air Act)
Regulate
Montreal Protocol (1980s) - an agreement originally signed by 24 countries (now 197 after 2000s) to regulate the production and consumption of CFCs
Started after a hole in the ozone layer was discovered over the Antarctic, reaching a peak size of 30 million square kilometres
by the 2000s, CFCs had been reduced by 30%
As of December 2024, the size of the ozone hole is 21.9 million km²
Restore
Despite research, no technologies have been implemented to restore the ozone hole
Formation of photochemical smog
Pollutants are released: nitrogen oxides [NOx] and volatile organic compounds [VOCs])
Sunlight hits them → causes chemical reactions
These reactions produce ozone (O₃) in the troposphere
In addition to ozone, the reactions also produce Nitrogen dioxide (NO₂), Peroxyacyl nitrates (PANs), Aldehydes & Fine particles
The mixture of these chemicals and ozone form photochemical smog
Thermal Inversions
a layer in the atmosphere in which air temperature increases with height.
Factors effecting smog formation
Topography → valley creates thermal inversions to trap smog
Population Density → More people, more pollution
Fuels types → used for industry, transportation and heating homes
Local climate → reductions by rain & snow cleansing the air or wind transporting pollution elsewhere
Pollution Management
Reduce
Decrease demand for electricity/fossil fuels by switching to renewable energy
Alter human activity → public transport, walking, biking, etc.
Regulate
Government regulation/taxation (carbon tax)
using catalytic converters to clean the exhaust of primary pollutants from car exhaust
Restore
adopting clean-up measures such as reforestation, regreening, and conservation
Acid Deposition and its sources
any form of precipitation with acidic components that fall to the ground from the atmosphere in wet or dry forms.
Sources:
coal burning power plants
smelters
cars
industrial plants
Types of acid deposition
Starting materials → Sulfur Dioxide (SO2) and Nitrogen Dioxide (NO2)
Wet deposition
SO2 and NO2 mix with water in the atmosphere to form nitric acid and sulfuric acid. it falls as rain, snow, vapour or fog and lasts 4-14 days
Dry deposition
Converted into particulate compunds as sulfates and nitrates. they last 2-3 days and falls near the emission source
Impacts of acid rain on plants, water/aquatic organisms and humans
Plants:
weakens tree growth (disturbs root growth & function)
lowers concentration of chlorophyll
breaks down lipids
needles shed more often
Water/Aquatic Organisms:
low biodiversity
kills indicator species
improved visibility
increased dissolved metals (zinc, lead, aluminium, etc.)
Humans:
respiratory diseases (bronchitis & asthma)
leach toxic materials (lead & copper) from pipes into drinking water
damages infrastructure
decreases atmospheric visibility
Management Strategies for Acid deposition
Replace
replace private vehicles with public transport, bikes & walking
switch to low sulfur fuels
use more renewable energy
Regulate
but caps on fossil fuels and offer subsidies for using renewable energy
Restoration
liming lakes - adding limestone (reduce acidification)