Handout 5 - Intro to Oxygen Binding
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
73 Terms
Is protein structure static?
No!
- Proteins are dynamic molecules
- Association with other molecules often involves changes in conformation
What other molecule do proteins often associate themselves with?
Ligands
What is one way ligands can bind to proteins?
Reversibly
What may ligands be?
small molecules, ions, other proteins/macromolecules, etc.
Where does binding occur?
specific 3D locations or binding sites/domains on the target protein
What do the binding sites/domains need to be to the ligand? Why?
Complementary
allows it to discriminate among 1000’s of molecules present in the environment!
What aspects are important in complementarity?
size, shape, charge, hydrophobic/hydrophilic character
The interaction between the ligand and the binding domain needs to have a high degree of what?
Specificity
so the domain only attracts the correct ligand(s) among the myriad molecules in the cell.
For different ligands, what might the protein have?
Separate binding sites
Binding may involve what element?
“Induced fit”
What is “induced fit”?
The idea that ligand binding can cause changes in the conformation or shape the protein, and this influences the function of the target protein
In multi-subunit proteins, if a conformational change occurs on one subunit, what happens?
Changes in the conformation of the other subunits will occur
What family are myoglobin and hemoglobin in?
the “Globin Family” of proteins
What example of proteins are myoglobin and hemoglobin?
Conjugated proteins
nonpeptide units absolutely needed for function, tightly associated with the protein, so they have prosthetic units (heme!)
What are protein families?
Proteins with similar primary sequence and/or 3D structure and function
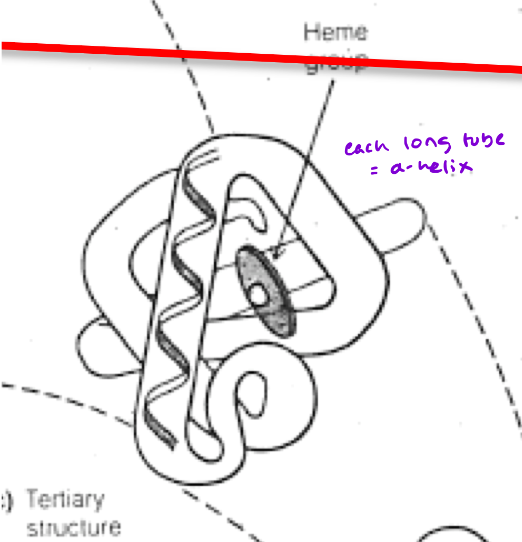
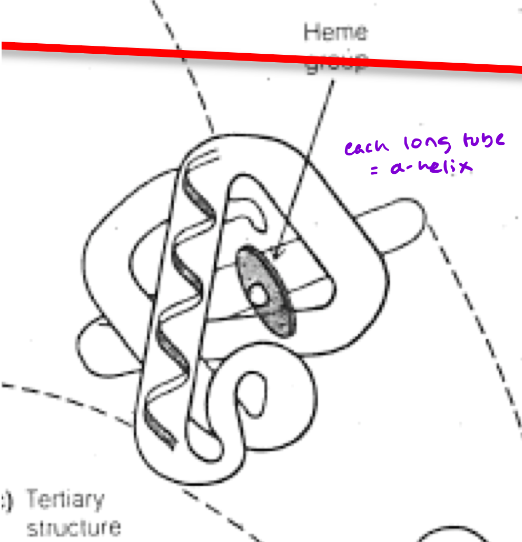
What type of protein (structurally) is Myoglobin (Mb)?
Monomeric protein
single polypeptide chain
3D structure is highly helical (75% alpha-helix, no Beta pleated sheets)
Where is Myoglobin found in the body?
Inside muscle tissue
skeletal and also heart
What critical role does Myoglobin play in our bodies?
It binds oxygen!
Important in oxygen storage!
Helping the tissue to take oxygen from the circulatory system and hold on to it until the muscle cells need the oxygen when the oxygen pressure/tension inside the muscle tissue runs low due to exertion.
What does Mb indicate?
De-oxy Myoglobin
What does Mb O2 indicate?
Oxygenated Myoglobin
What does each Myoglobin molecule have?
A single O2 binding domain
This allows it to reversibly bind a single oxygen molecule.
What type of protein (structurally) is Hemoglobin (Hb)?
Tetrameric = 4 subunits
(alpha2, Beta2)
2 alpha subunits have identical structure
2 Beta subunits have identical structure
alphas and betas are very similar in their primary, secondary, and tertiary structures
Where is hemoglobin found in the body?
It is the major cytosolic protein in red blood cells (RBC’s).
Its the red color of hemoglobin that gives these cells their characteristic red color.
What are the jobs of hemoglobin?
Oxygen transport
Because hemoglobin is in RBC’s, and these move through the circulatory system, we have a perfect way to move oxygen from the lungs — where de-oxy hemoglobin picks up oxygen to become oxy hemoglobin — and have this move through the tissues where it releases oxygen to be picked up by myoglobin.
Oxy hemoglobin is turned back into de-oxy hemoglobin and then the red cells make the transition back to the lungs for another pick up.
De-oxy Hb —> Oxy Hb
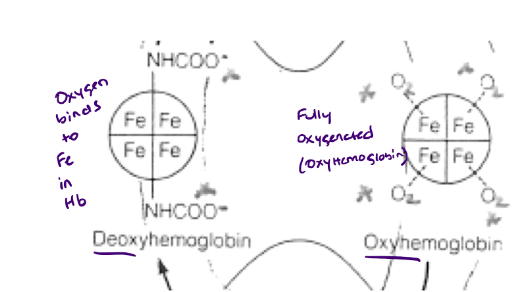
How many subunits are on a single hemoglobin? What does this mean?
4 subunits
Each one has a separate O2 binding domain (or heme), so while myoglobin only picks up one O2, hemoglobin picks up 4.
What is hemoglobin in the transport of oxygen?
A key molecule!
What type of protein (figuratively) is myoglobin?
A storage protein!
What is Dr. B’s favorite cell?
Red Blood Cells (which contain hemoglobin)
What are the 3 reasons we need proteins to associate with O2 to transport it in the body and to store it in the tissues?
Poor diffusion in tissues
Need to regulate transport (i.e - respond to changes in the environment or stress)
Greatly increases O2 solubility in water
How much does the oxygen solubility increase when using Hemoglobin as the transporter instead of using water?
~100 fold
Does O2 bind to the protein itself?
No, it binds to a prosthetic unit.
What is a prosthetic group or unit?
Nonpeptide unit
tightly associated with the protein
absolutely required for function
How do we see prosthetic groups in Mb and Hb?
Globin: the protein part
Heme: the prosthetic group
Why do we need Heme?
None of the amino acids are suited to bind oxygen
What do all of the species (structures of myoglobin and the beta subunit of hemoglobin) have?
The classic “globin fold”
What exactly is the classic “globin fold?”
8 alpha helices, 7 bends, 75% alpha helical
Very compact (not a lot of free space in the core)
Interior nearly all Hydrophobic amino acids [except for 2 interior histidine residues (critical for function)]
The single polypeptide chain of Mb and each (4) subunit of Hb have a heme
Located in nonpolar crevices of the protein
What communication occurs within Myoglobin molecules?
Each Mb molecule acts independently (no communication between hemes of different Mb molecules)
What does a fully oxygenated hemoglobin have?
4 oxygen molecules bound
What communication occurs within Hemoglobin molecules?
Between the hemes on the different subunits of a Hb tetramer
What two things are similar within the globin proteins?
Similar subunit structure (globin fold) and heme association
What is hemoglobin said to be?
A “dimer of dimers”
The linkages between the different subunits are not exactly the same.
What do you mean by “dimer or dimers”?
alpha1beta1 and alpha2beta2: subunits in these dimers are tightly linked; the 4 subunits can “communicate” with each other through subunit contacts
What does O2 binding at hemes cause?
Causes changes at the alpha1Beta1 and alpha2beta2 subunit interfaces which changes O2 binding affinity
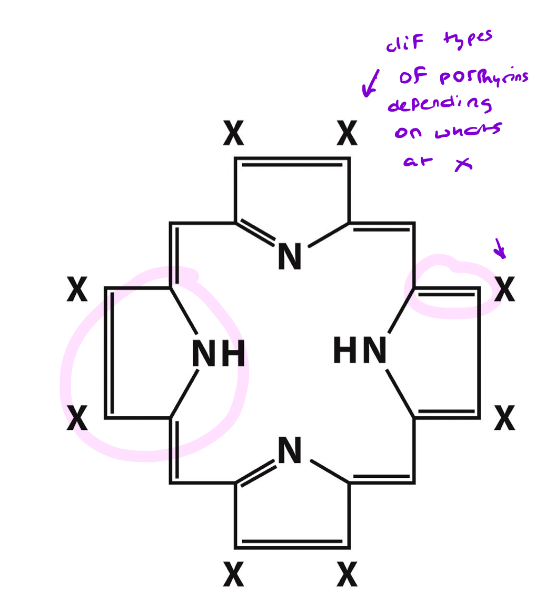
What is Heme?
A complex organic ring structure based on a porphyrin
porphyrin in heme = protoporphyrin 9
Structurally, what is a porphyrin?
An organic molecule that has 4 pyrrole rings linked by methene bridges
Different porphyrins have different substituents at the X positions
Nonpolar; fits in a nonpolar crevice in the protein
Ring system is somewhat planar
What is the heme in Mb. Hb (oxygen carriers)?
A coordination compound with a bound iron atom
What must the oxidation state of the iron bound to oxygen carriers be?
2+
Must be ferrous! “ferroHb”
Color will be orange-red
If not, the heme is no longer able to reversibly bind oxygen, it’ll bind water instead
Oxidation state = 3+ (ferric) ; color will be brown; “ferriHb”
What are 4 of the coordination sites on the iron occupied by? What about the 5th and 6th site?
By the pyrrole Nitrogen
The 5th site = linkage to a nearby histidine (proximal histidine); covalent bond
The 6th site = O2 binds to the heme and thus to the oxygen binding protein
What does O2 binding to 6th coordination site of iron cause?
Binding causes iron to move into plane of porphyrin ring
What other ligands other than oxygen can serve as Lewis-Bases to bind to the electron poor iron?
CO, NO, CN-, etc.)
What does the proximal histidine on heme do?
Creates a 6th link to iron
What does the distal histidine on heme do?
In a position just above where the oxygen binds
When no oxygen is bound, what happens to the iron in the heme?
Iron is actually displaced slightly (0.3 Å) above the plane of the porphyrin when no ligand (oxygen) is bound at the 6th coordination site.
What roles does the proximal histidine have?
Covalent link to heme (when the iron moves, so does the proximal histidine)
What roles does the distal histidine have?
Prevents strong CO binding
other roles too (i.e - H-bond to O2)
What’s an additional role for globin? (in terms of binding with O2)
Involves the ability of another ligand to compete with O2 for binding: carbon monoxide
How much tighter can carbon monoxide bind to the iron as opposed to oxygen?
CO binding to isolated heme: 20,000 x tighter than O2
CO binding to heme in Hb: 200 x tighter than O2
What is it about the globin that decreases the CO binding affinity to heme?
O2 may bind in preferred bent geometry (optimal binding affinity)
CO may not bind in preferred linear geometry (thus lowered binding affinity)
What’s another role of globin?
To prevent oxidation of the heme iron
How can we ensure that the tendency of the iron to become oxidized is very limited?
Provide an enzyme that will reduce metHb when it forms the enzyme metHb reductase
Cleverly engineer the structure of the globin so that iron is much less likely to oxidize
Overall, what’s our premise regarding globin? How can we look at this?
It helps to inhibit oxidation of the iron
To see this, we would need to look at heme molecules in the absence of any globin.
In order for oxidation to occur, what needs to be formed?
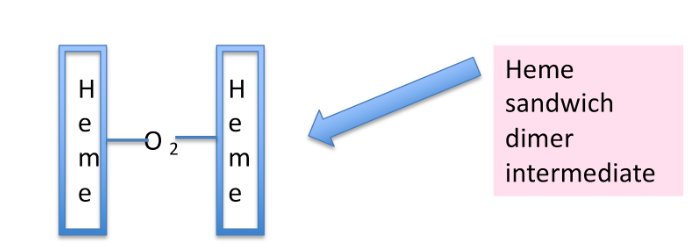
A “sandwich dimer” intermediate
What is a “sandwich dimer” intermediate?
Two hemes must get close enough (without obstruction)
Two hemes bridged by an oxygen molecule (so heme is the bread, oxygen is the filling in the sandwich)
What are the synthetic Heme molecules that were designed to prevent iron oxidation called?
“Picket fence” Porphyrin
Porphyrin ring
4 pyrrole Nitrogen forming the 4 coordination links to the iron atom
Imidazole molecule forming the 5th coordination link (mimicking R-group of histidine)
Ring had big bulky substituents built into the structure
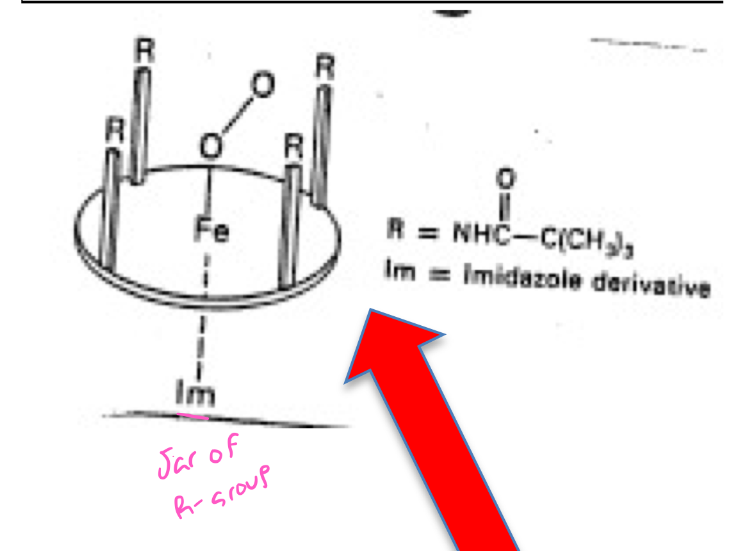
What did the researchers find out about their “picket fence” porphyrin? Did it prevent or allow O2 binding?
O2 binding is similar to that in Mb
What did the researchers find out about their “picket fence” porphyrin? Did it allow or prevent the iron from being oxidized?
Iron remains in reduced form (not true for hemes without the picket fence!)
If a protein (P) interacted temporarily with another molecule (L) what would that give?
A reversible, transient chemical equilibrium
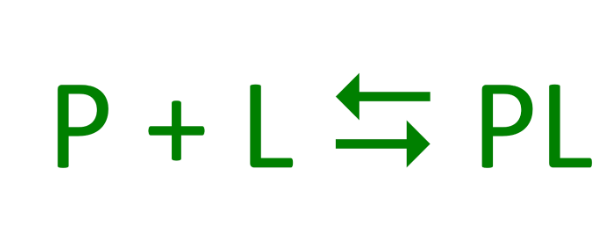
What is the molecule that binds to the protein called?
Ligand (typically a small molecule)
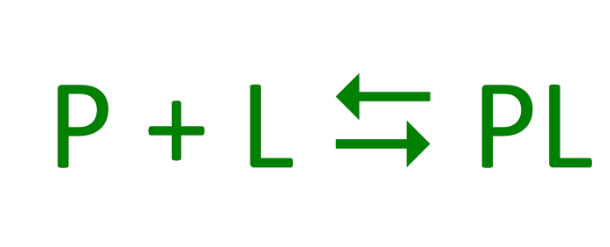
What is the region in the protein where the ligand binds called?
The binding site
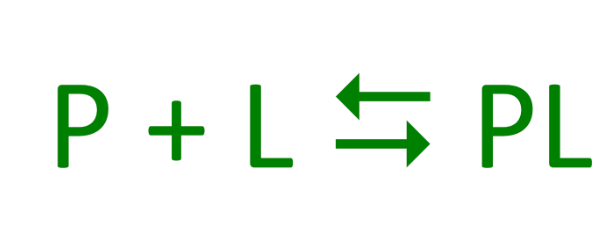
How does the ligand bind to the protein?
Via the same noncovalent interactions that dictate protein structure
This allows the interactions to be transient and reversible
How can the process in which a ligand (L) binds reversibly to a site in a protein (P) be quantitatively described?
By the association rate constant (ka)
By the dissociation rate constant (kd)
After some time, the process will reach the equilibrium where the association and dissociation rates are equal
What is the equilibrium composition characterized by?
The equilibrium association constant (ka) or the equilibrium dissociation constant (kd)
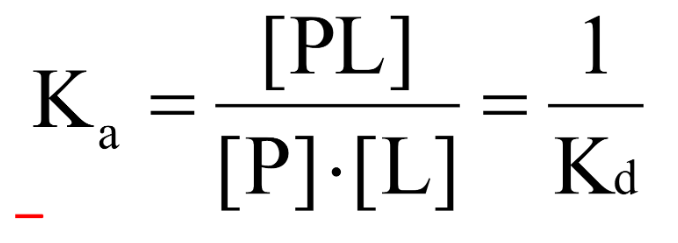
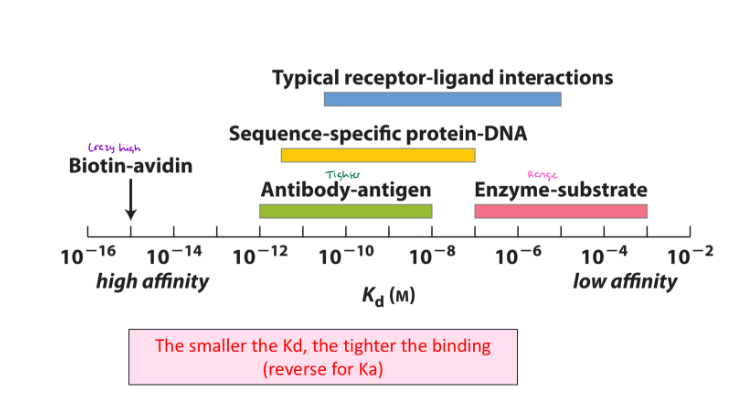
The smaller the Kd (or the larger the Ka)…
The tighter/greater the binding affinity will be