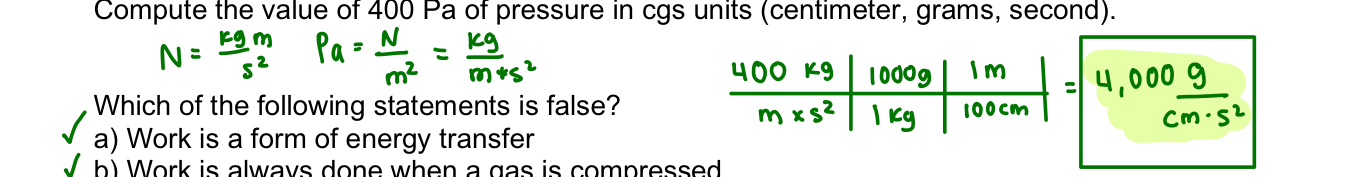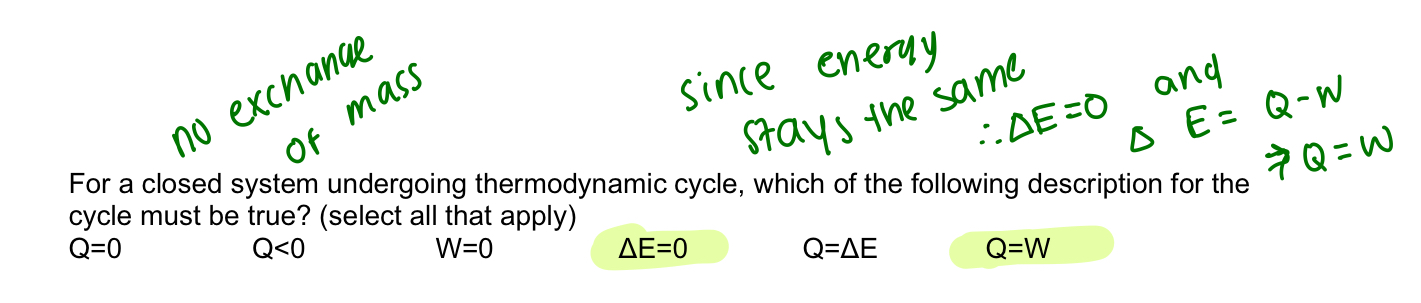thermofluids practice
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms




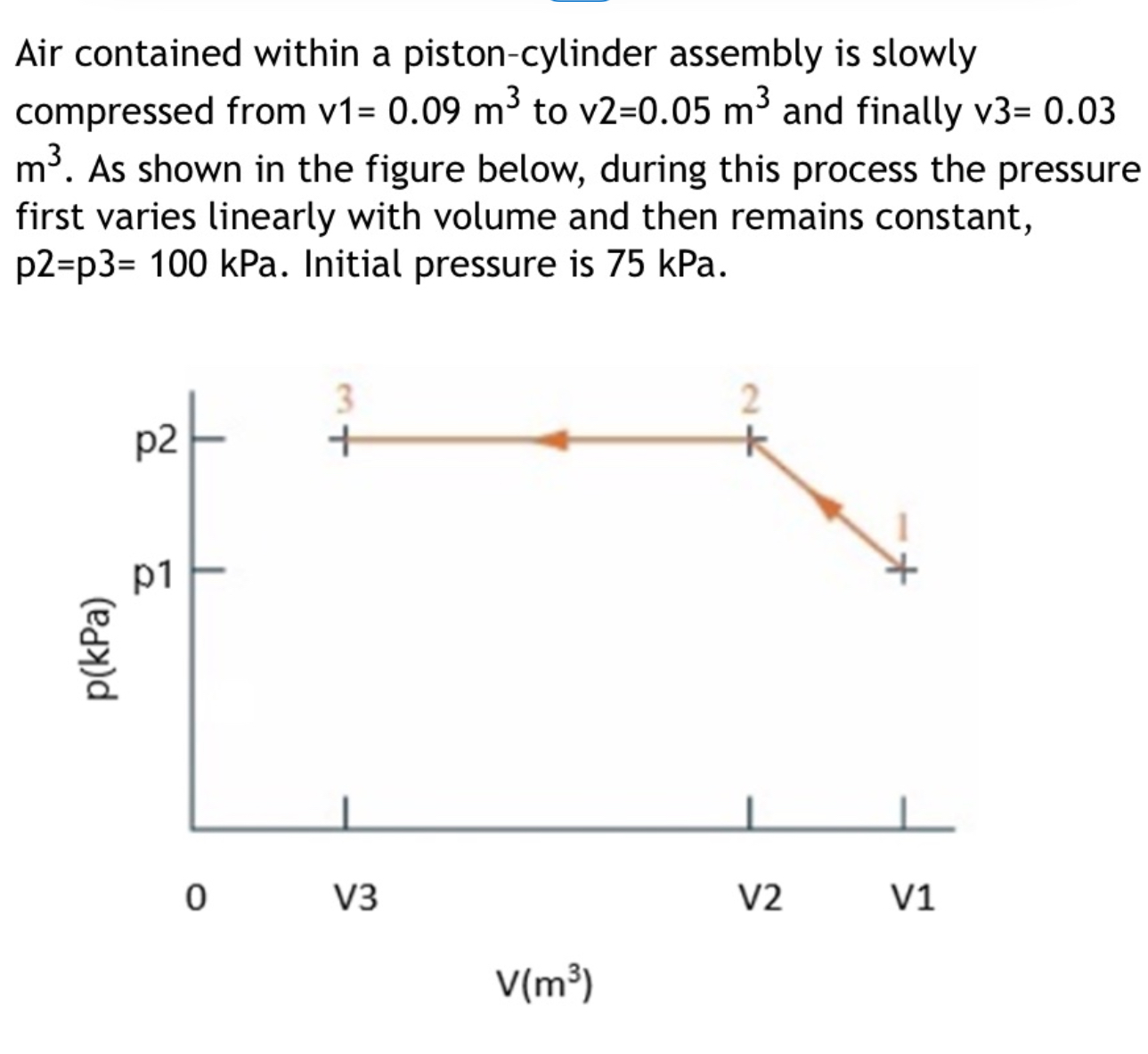
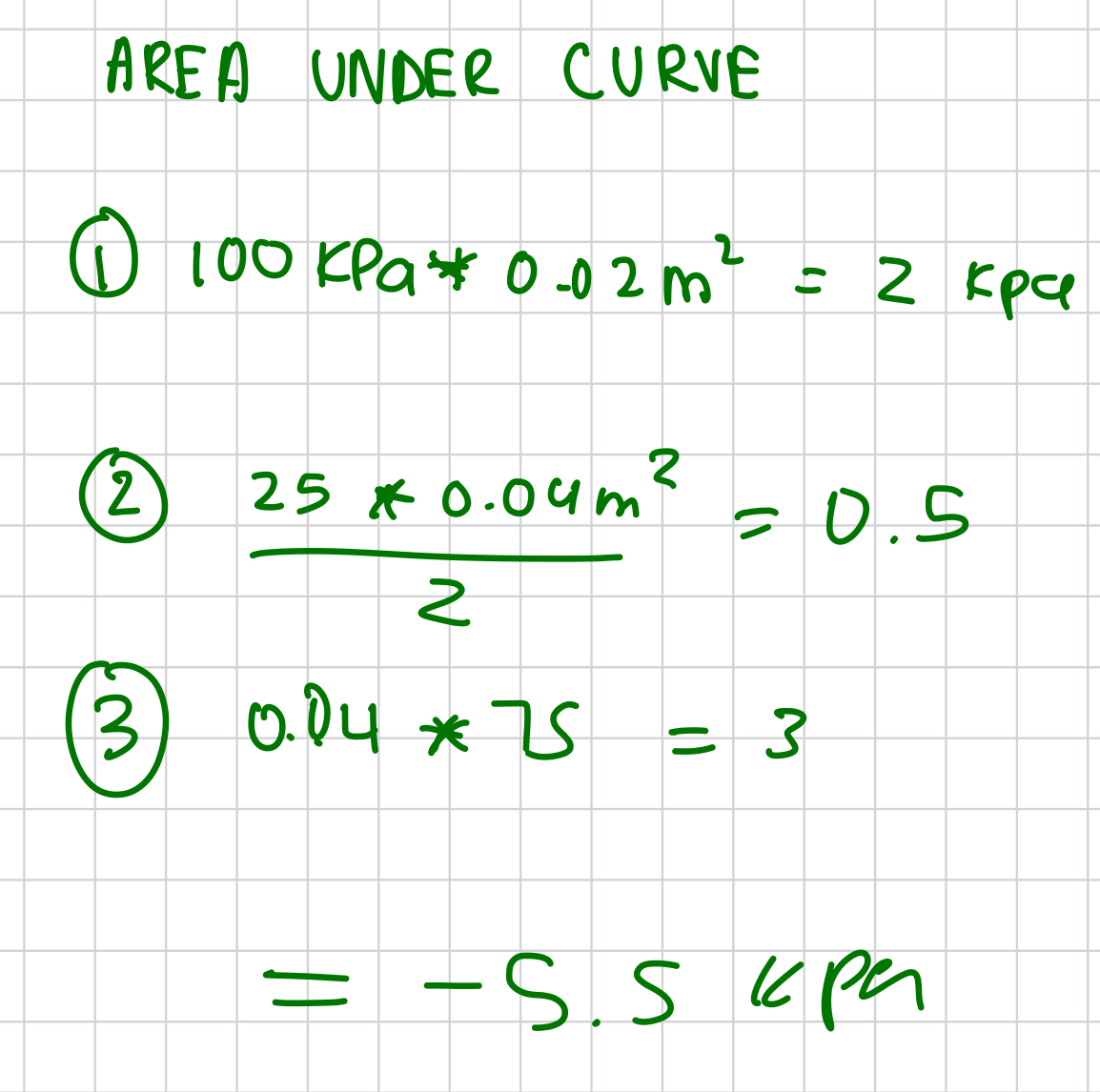
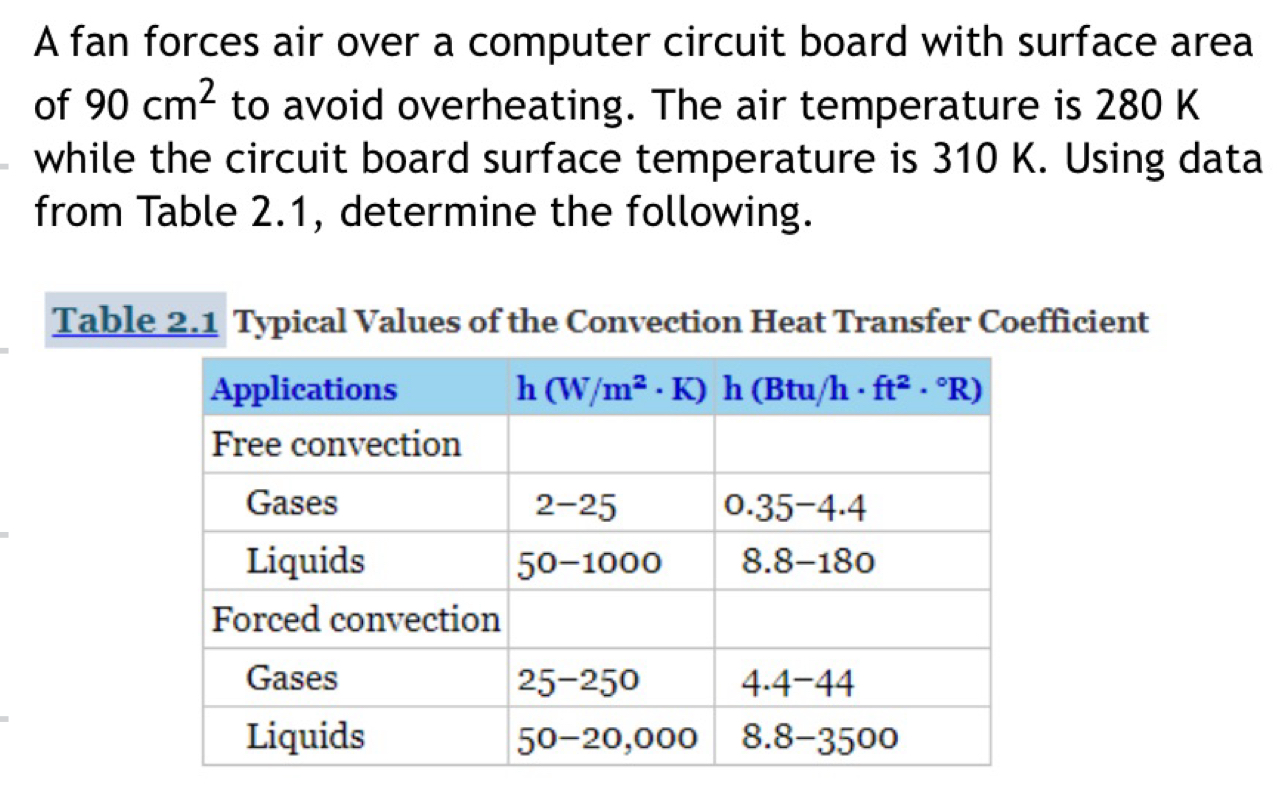
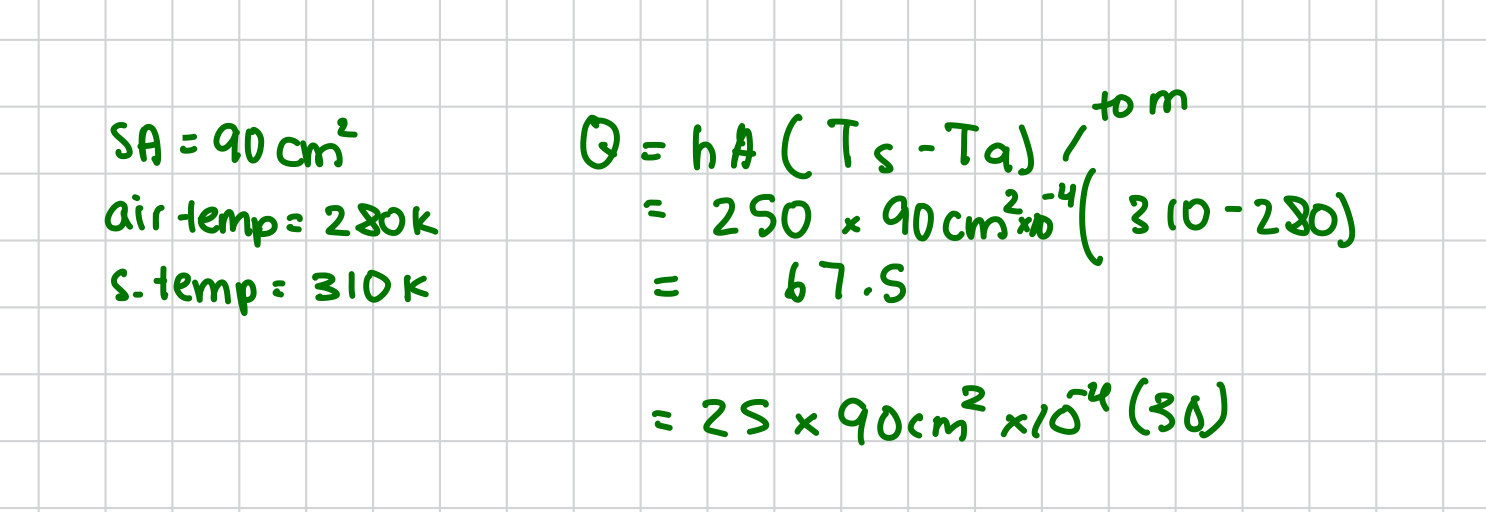

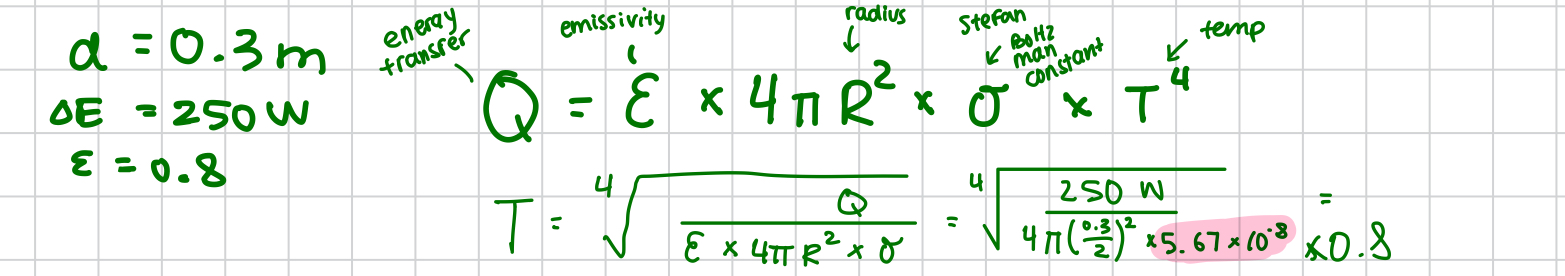

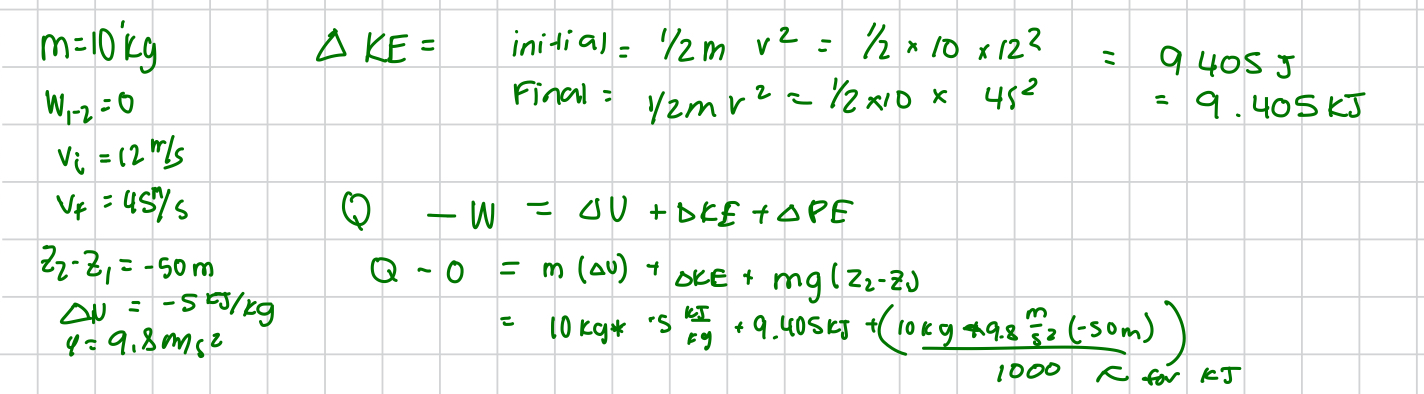
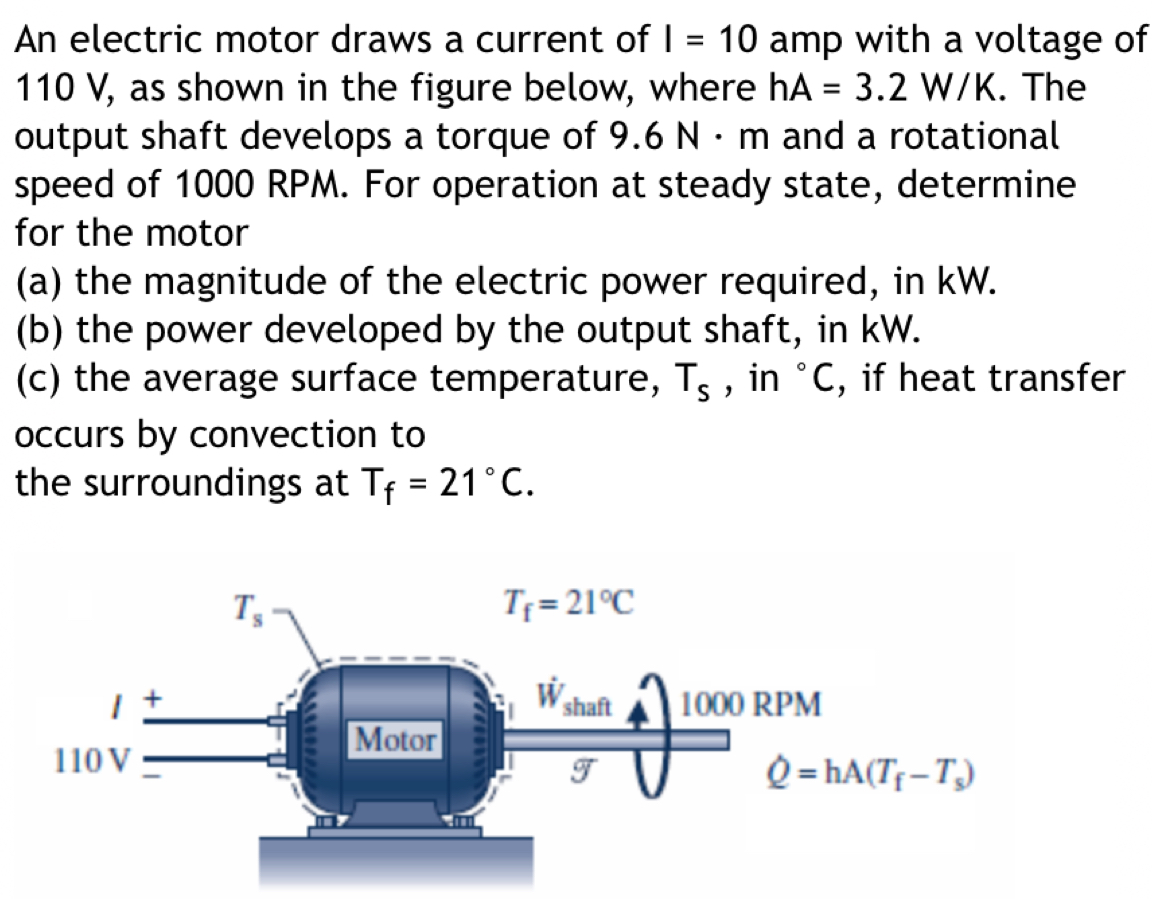
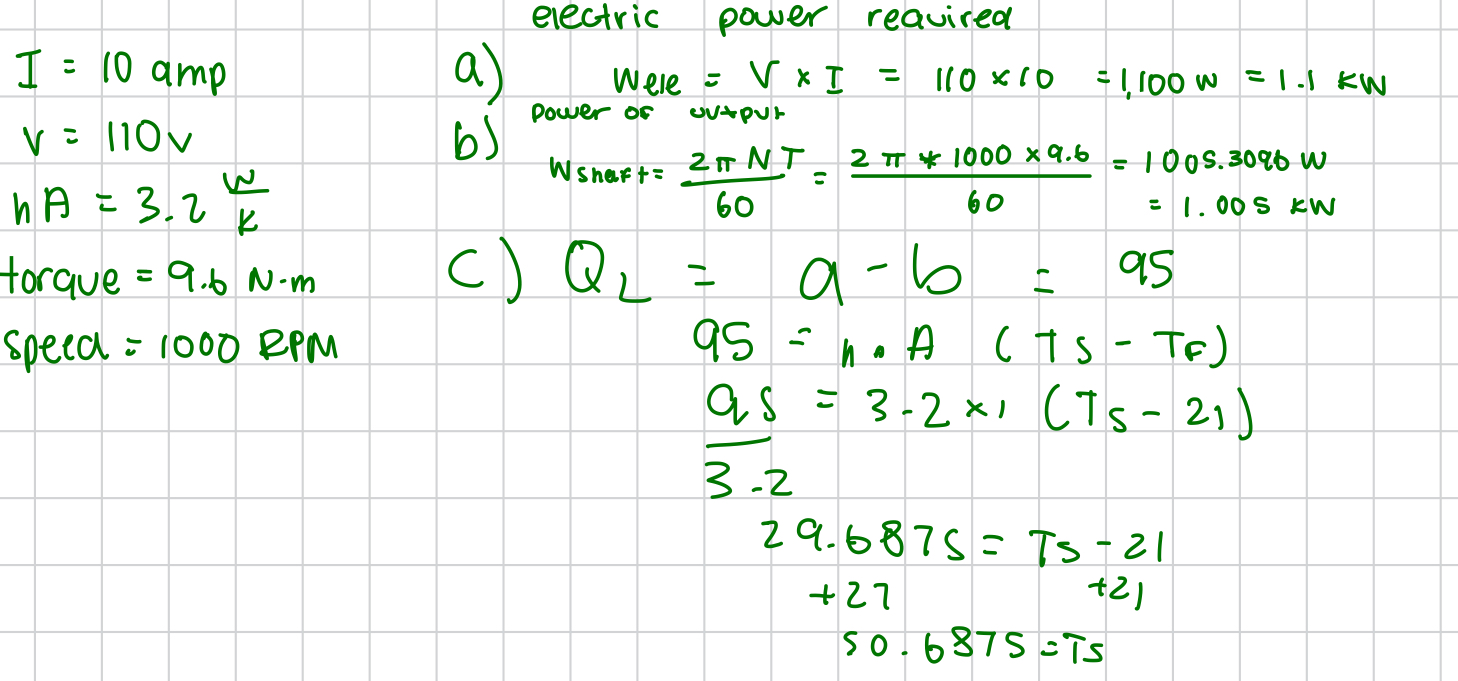
stefan boltzmann constant
5.67 ×10^-8
extensive property
dependent on the amount
intensive property
not dependent on the amount
examples of intensive properties

examples of extensive properties

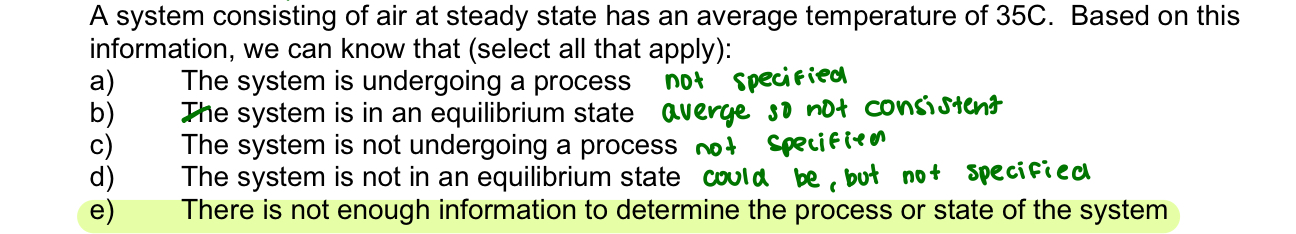
steady state
system variables stay the same overtime
equilibrium state
the same throughout the system
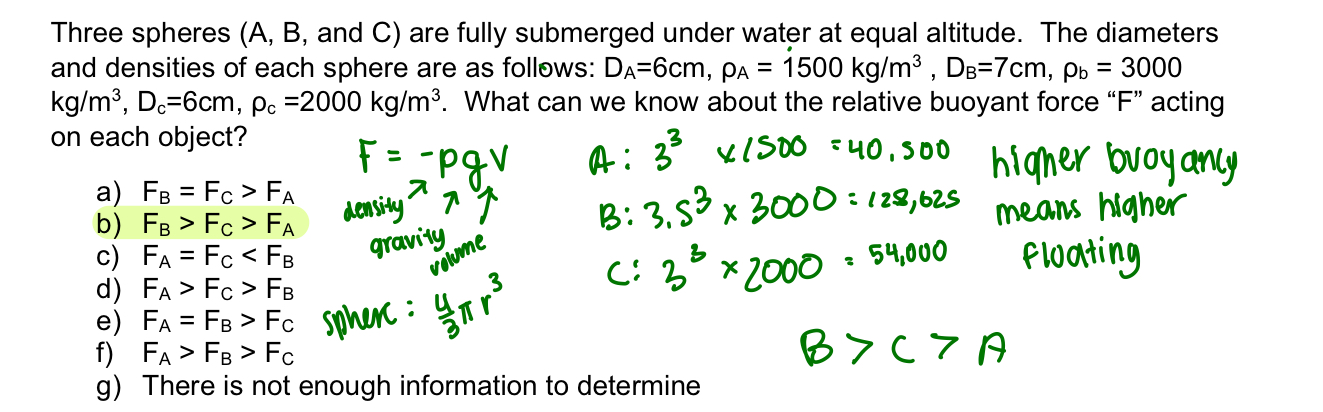
buoyant force equation
buoyant force= -pgv
higher buoyancy?
floats more
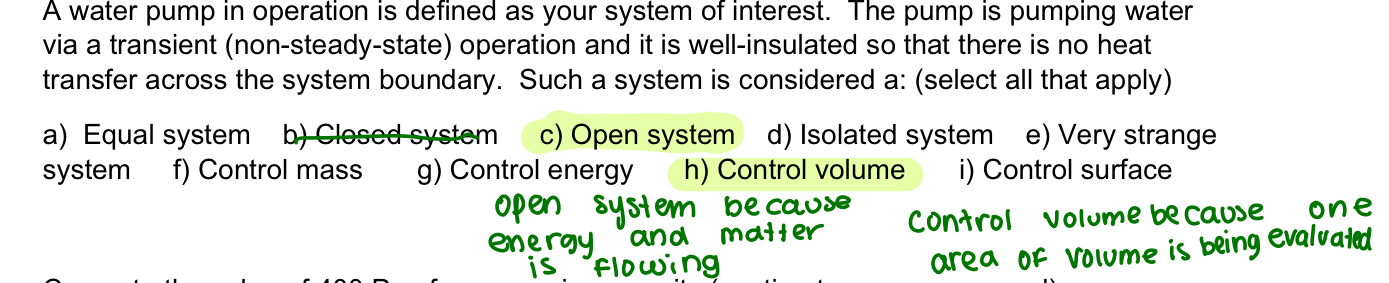
closed system
can exchange energy but not matter (control mass)
open system
exchange of energy and mass (control volume)
boundary also known as?
control surface
isolated system
no exchange of energy or matter
property
macroscopic characteristic of a system independent of the previous behavior (history) of the system
state
the condition of a system as described by a set of properties
process
transformation from one state to another
lb m to lb f
1 lb f= 32.17 lbm
one atmosphere
1 ATM = 101.3 kPa or 14.7 lbf/in2
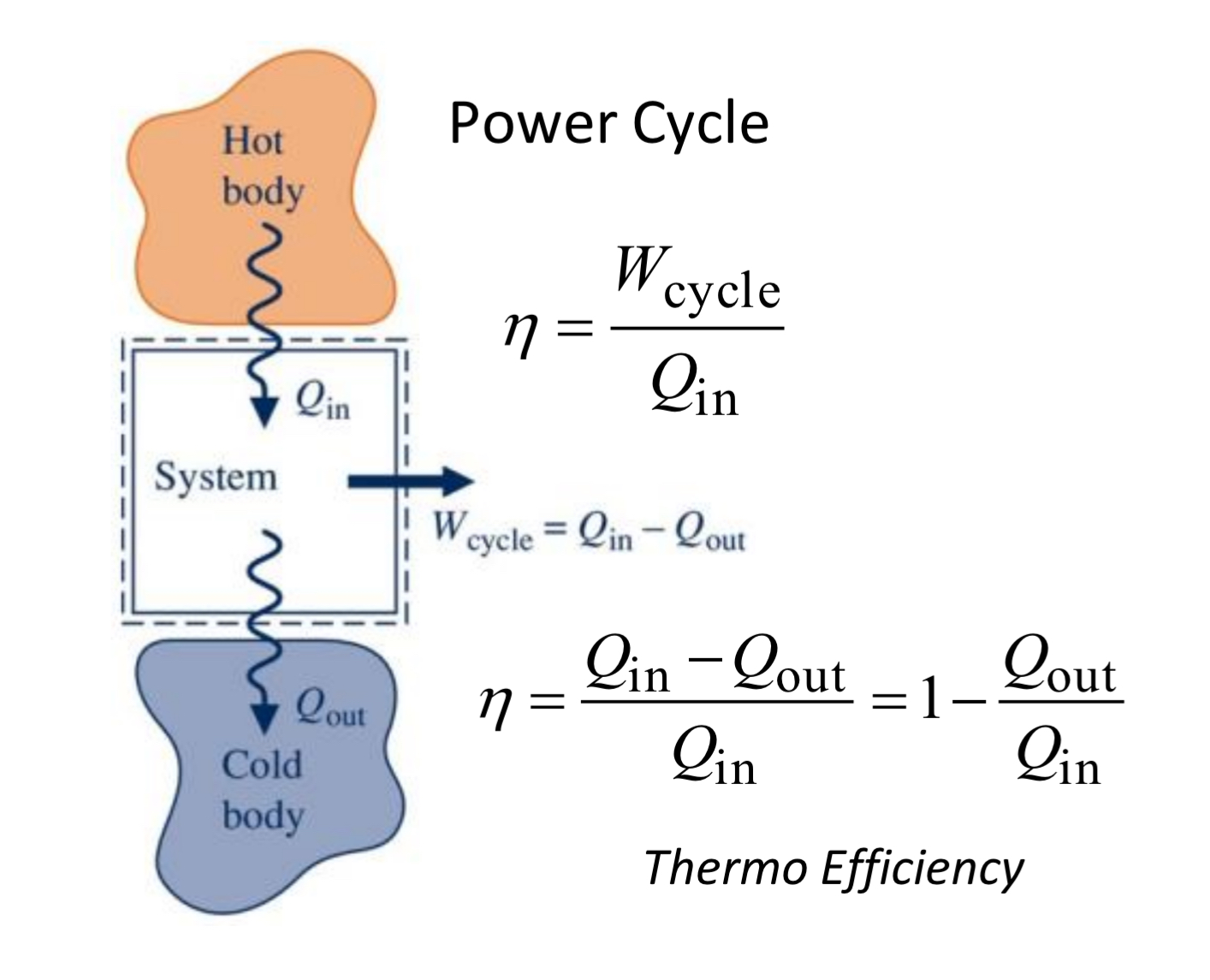
power cycle
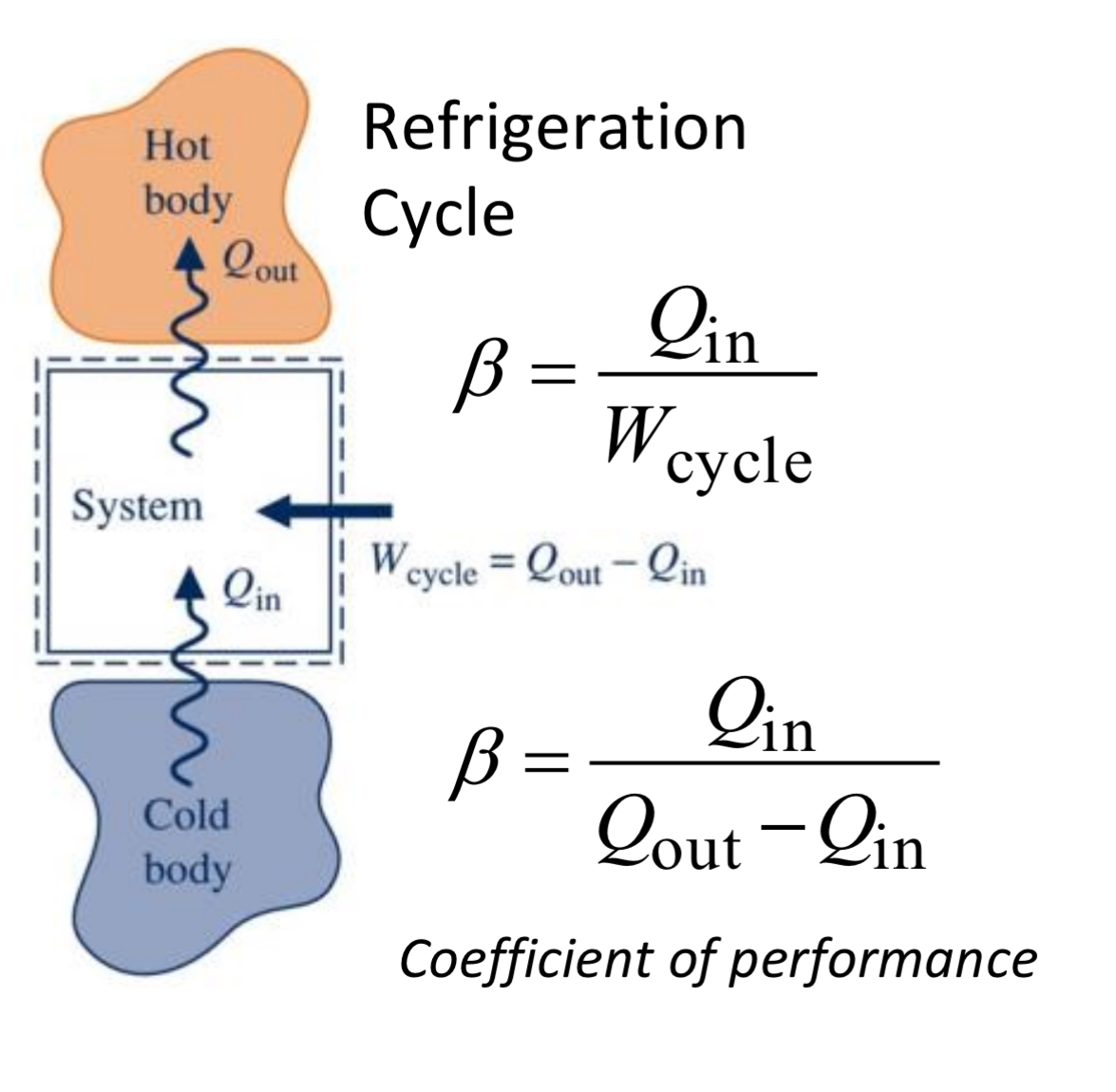
refrigeration
heat pump
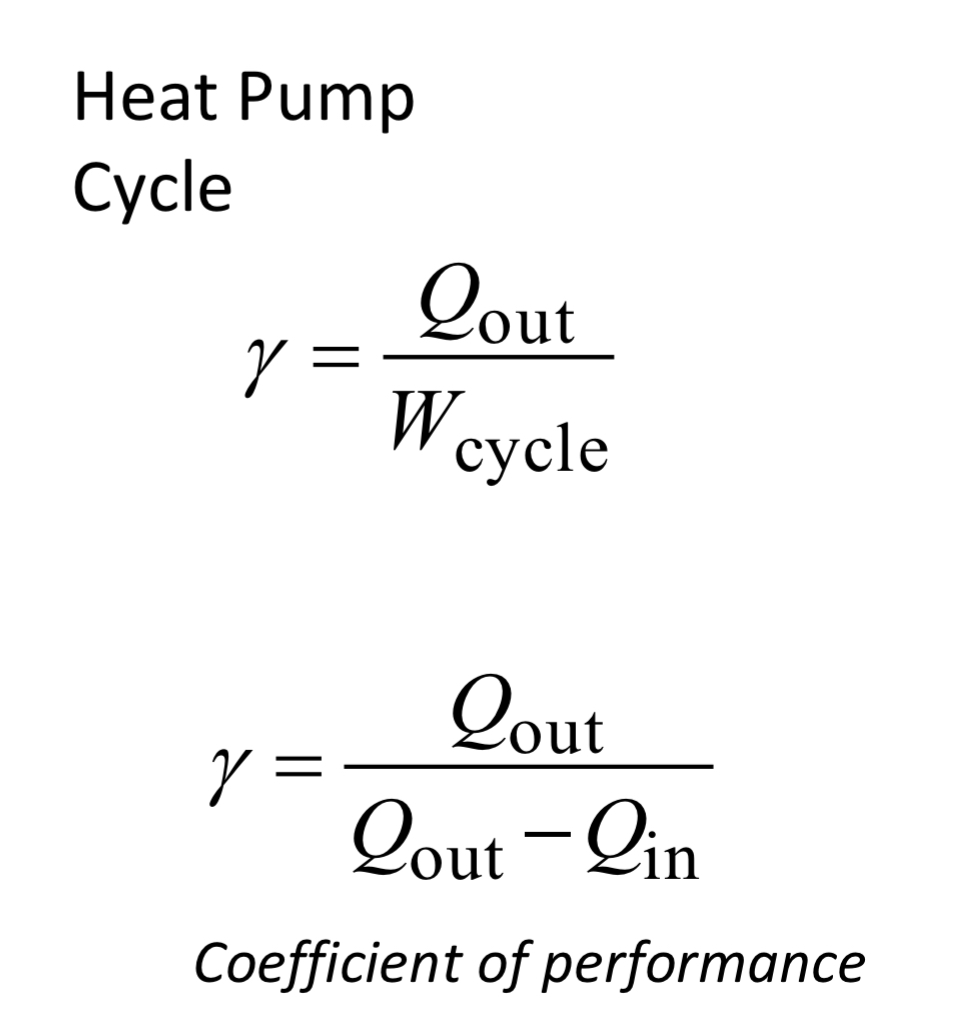
quasi-equilibrium
A process that occurs slowly enough for the system to stay close to equilibrium at all times.