alcohol reactions
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Primary Alcohol with Hydrogen Halide (H-X)
The OH gets protonated as LG and follows SN2 mechanism with H2SO4
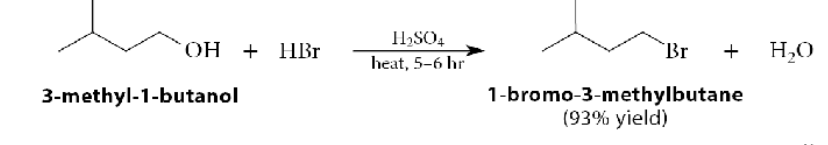
Secondary and Tertiary Alcohol with Hydrogen Halide (H-X)
Follows SN1 mechanism and is faster because of resonance stabilization
Primary and secondary Alcohol reactions with SOCl2 and PBr3
OH gets protonated as a LG and follows SN2 mechanism with ether
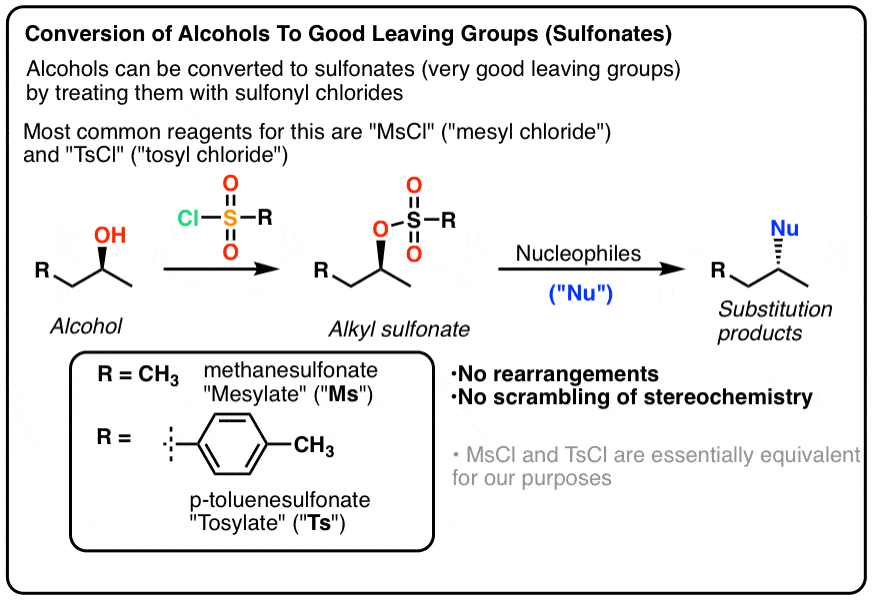
Conversion of Alcohol into a Sulfonate Ester (good LG)
Alcohol with MsCl (CH3-SO2Cl) or p-TosCl/Pyridine turns it into good LG to undergo SN2 mechanism (inversion)
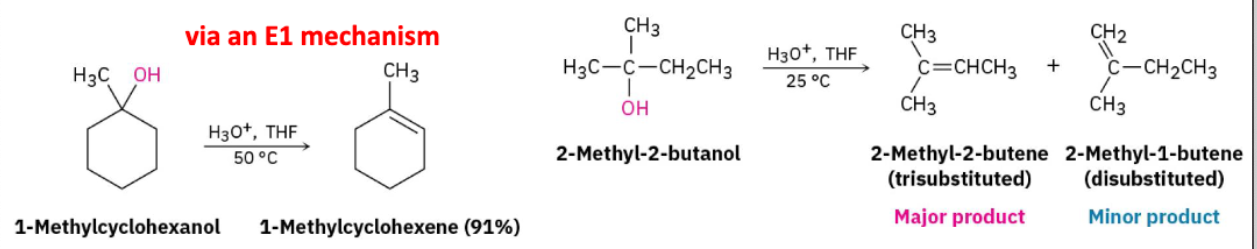
Dehydration of Alcohols w/ H3O+, THF
Alcohol undergoes E1 mechanism to form alkene
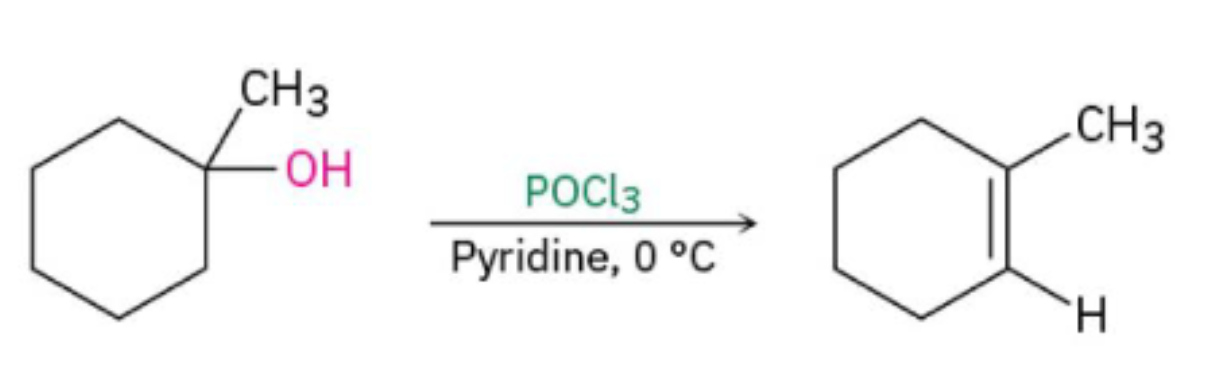
Dehydration of Alcohols w/ POCl3/Pyridine
Alcohol undergoes an E2 mechanism to form alkene
Oxidation of primary alcohol
produces an aldehyde
Oxidation of an aldehyde
produces a carboxylic acid
oxidation of a secondary alcohol
produces a ketone
Oxidation of a tertiary alcohol
produces no reaction
Example of mild oxidants and their product
PCC gives an aldehyde from alcohol
Dess-Martin gives an aldehyde or ketone
Example of strong oxidants and products
KMnO4 and H2CrO4 produce carboxylic acids from alcohol
Substitution with alkyl halide
primary R-X with strong base (NaOH) → primary alcohol
tertiary R-X with weak base (H2O) → tertiary alcohol
Reduction of Carbonyl
carbonyl with NaBH4 or LiAlH4/H3O+ → alcohol
Addition with alkene
Hydration: H3O+ → markovnikov alcohol
Hydroboration: (1. BH3 THF 2. H2O2, NaOH) → anti-markovnikov alcohol
Halohydration: (X2/H2O) → markovnikov alcohol with X
Grignard reagent with carbonyl compound
R-X + Mg → R-MgX (Grignard reagent)
carbonyl with
1. RMgX, ether
2. H3O+
→ alcohol + HOMgX