Chemistry - C6 - Organic Chemistry
1/84
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
85 Terms
organic chemistry refers to compounds containing which element?
carbon
what is a hydrocarbon?
a compound containing only carbon and hydrogen atoms
name the first four alkanes
methane (CH4)
ethane (C2H6)
propane (C3H8)
butane (C4H10)
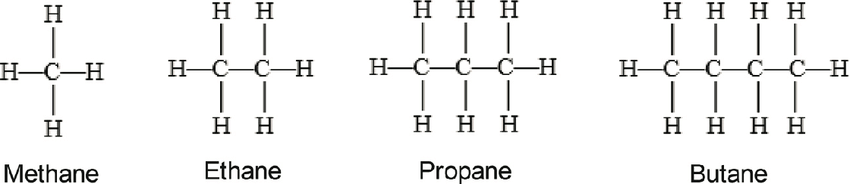
what is the name for CH4
methane
what is the chemical formula for ethane?
C2H6
how many carbon atoms are in a molecule of propane?
3
what is the name of the alkane with four carbon atoms?
butane (C4H10)
what is the name for a compound with no double bonds?
a saturated compound
all alkanes are saturated compounds
what is the general formula for an alkane?
CnH2n+2
what do properties of hydrocarbons depend on?
the length of their carbon chain
for alkanes, what happens to boiling points as length of chains increase?
boiling points increase
the first four alkanes are gas at room temp due to their low boiling point
shorter alkanes are more (…) than longer alkanes
name three possible answers
lower boiling points
more volatile (evaporate more easily)
more flammable
therefore better fuels
as the chain length of alkanes increases, do they become more or less viscous, volatile and flammable?
more viscous
less volatile
less flammable
what is the main use of hydrocarbons and why?
for fuel
because they release lots of energy when burnt with oxygen
as long as there is sufficient oxygen when burning a hydrocarbon, what occurs?
complete combustion
hydrocarbon + oxygen → carbon dioxide + water
is combustion an exothermic or endothermic reaction?
exothermic
what is oxidised during complete combustion?
hydrogen and carbon
creating water
what is a homologous series?
a group of chemicals which have similar chemical properties and can be represented by a general formula
what is the functional group in an alcohol?
-OH
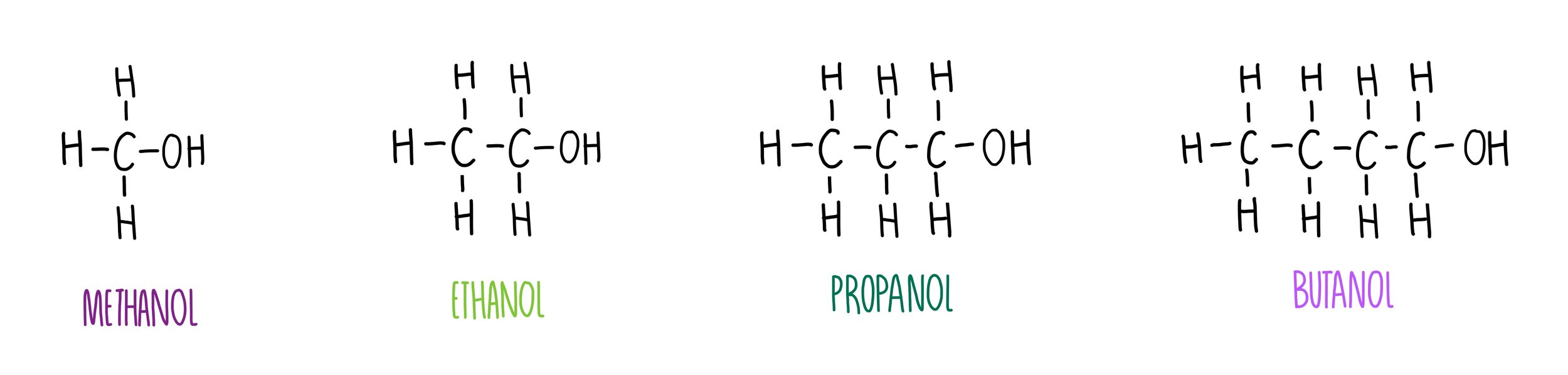
what is the general formula for alcohols?
CnH2n+1OH
are alcohols hydrocarbons?
no
they contain oxygen
what are the first four alcohols?
methanol (CH3OH)
ethanol (C2H5OH)
propanol (C3H7OH)
butanol (C4H9OH)
what three properties do the first four alcohols share?
flammable
soluble
can be oxidised to form carboxylic acids
why are alcohols often used as solvents in industry?
they can dissolve things that water can’t
eg. hydrocarbons, lipid compounds (fats and oils)
note: alcohols can also be used as fuels as they release lots of energy
what is the functional group for carboxylic acids?
-COOH
what is the general formula for carboxylic acids?
CnH2n+1COOH
how are carboxylic acids made?
by oxidising an alcohol (using an oxidising agent)
what are the names of the first four carboxylic acids?
methanoic acid (CH3COOH)
ethanoic acid (C2H5COOH)
propanoic acid (C3H7COOH)
butanoic acid (C4H9COOH)
are carboxylic acids weak or strong acids?
weak acids
don’t fully ionise
what do the negative ions formed by the (partial) ionisation of carboxylic acids end in?
-anoate
eg. propanoic acid ⇌ propanoate ions + hydrogen ions
what is the symbol equation for the ionisation of ethanoic acid?
C2H5COOH ⇌ C2H5COO+ + H-
this is a reversible reaction
the negative ion formed is ethanoate
what is formed when you react a carboxylic acid with a metal carbonate?
carboxylic acid + metal carbonate → salt + water + carbon dioxide
carboxylic acids react like any other acid
what homologous group are used for addition polymerisation?
alkenes
what is the key feature of an alkene?
carbon-carbon double bond
this makes alkenes unsaturated
what does the equation for an addition polymerisation look like?
draw bonds of monomer directly above and below carbon-carbon double bonded atoms
simplify complex groups into their formula
draw empty bonds of repeating unit going through brackets at sides
remember ‘n’s - same number on both sides
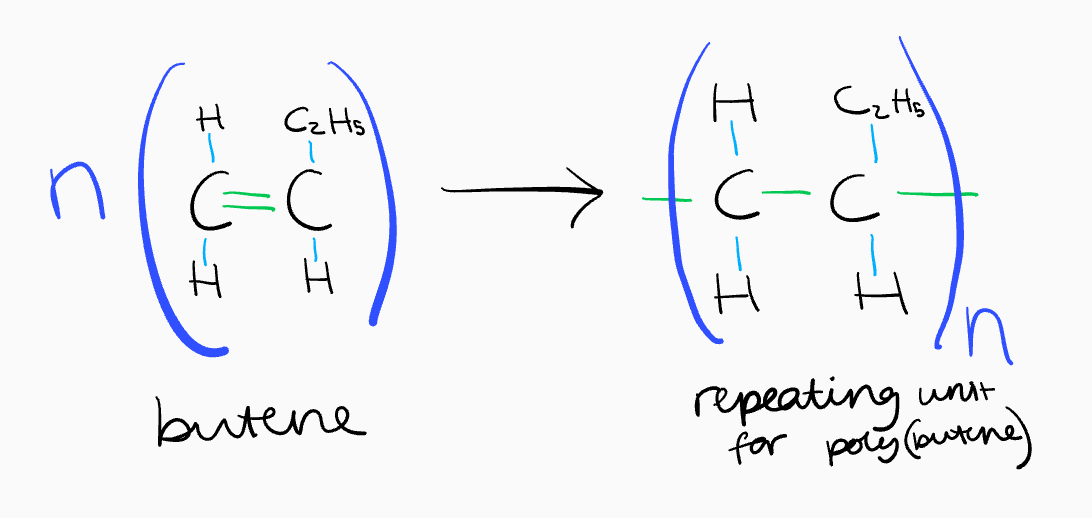
how do you know the name of an addition polymer?
the name is poly(monomer)
eg. poly(ethene), poly(butene), poly(chloroethene)
what conditions are required for addition polymerisation?
high pressure
catalyst present
condensation polymers can be made by joining together which two monomers?
dicarboxylic acid monomers (contain 2 carboxylic acid groups)
diol groups (contain 2 alcohol groups)
these are joined together by ester links
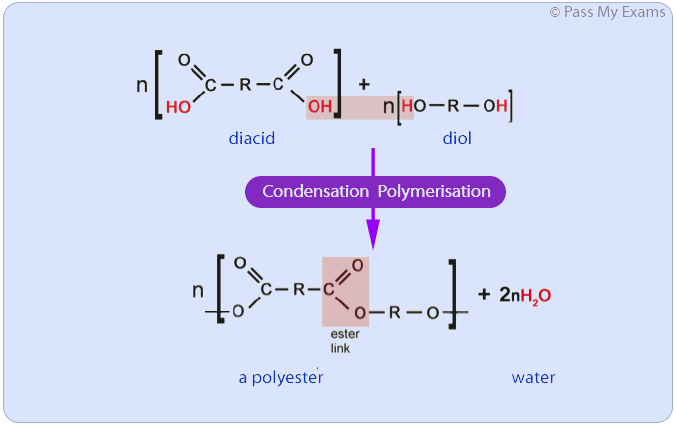
for every ‘n’ repeating units, how many water molecules are produced during condensation polymerisation?
2n
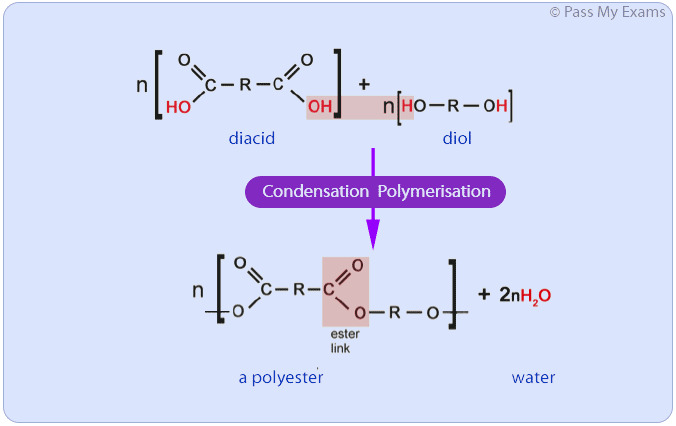
for molecules to be able to combine in condensation polymers, what two things must be true?
each of the monomers must have at least two functional groups
there must be at least two different functional groups overall
a small molecule is always given off during condensation polymerisation; what is this most often?
water
why are polyesters typically biodegradable?
microorganisms can break down the ester links
which type of polymerisation results in a non-biodegradable polymer?
addition polymerisation
what is the general formula of alkenes?
CnH2n
what is a dimer?
two monomers combined
when is a polymer referred to as a 'condensation' polymer?
when water is produced as a by-product of the reaction
what are polypeptide monomers called?
amino acids
what are dna monomers called?
nucleotides
what are carbohydrates made up of (monomers)?
sugars (monosaccharides)
what do polypeptides fold up, or combine, into?
proteins
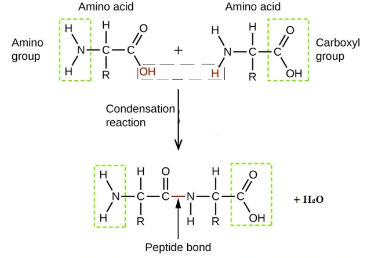
which three functional groups are present in an amino acid (monomer)? which is not used for condensation reactions?
amino group (left)
carboxyl group (aka carboxylic acid group, right)
R group (not used in condensation reactions)
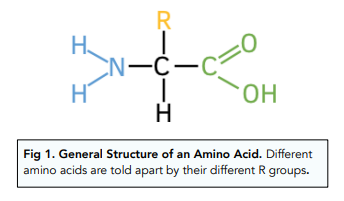
what distinguishes different amino acids?
their ‘R’ group
what type of reaction combines amino acids into a polypeptide?
condensation polymerisation
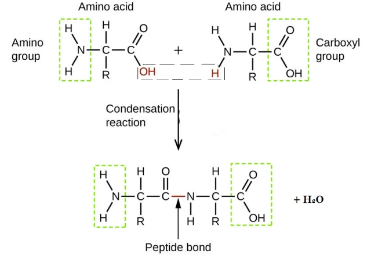
what other names are there for a peptide bond, and where is this bond found?
amide bond
amide link
between carbon and nitrogen in a polypeptide
how do we prevent the dna from getting damaged?
DNA is made up of 2 polymer chains that link together (naturally coiling around each other into a double helix shape)
what do all carbohydrates provide?
energy
refers to a number of different monomers and polymers
what are carbohydrate polymers called and what are some examples?
polysaccharides
eg. starch, cellulose, glycogen
what are carbohydrate monomers called and what are some examples?
monosaccharides, aka sugars
eg. glucose, fructose
what are most of the compounds in crude oil?
hydrocarbons (typically alkanes)
how are crude oils formed, and extracted from the ground?
crude oil is formed naturally from the remains of dead plants/animals (especially plankton) millions of years ago
the crude oil soaked into rocks and was stored
it can be removed by drilling into the rocks and extracting it
what is fractional distillation used for, in terms of crude oil?
separating the different compounds within crude oil, by making use of their different boiling points
how does fractional distillation of crude oil work?
feed oil into a chamber - heat until gaseous
pass the gaseous mixture into a fractionating column (hot at the bottom, cooler going up)
the gas rises up the column, until it reaches a temperature below its boiling point, then condenses into a liquid
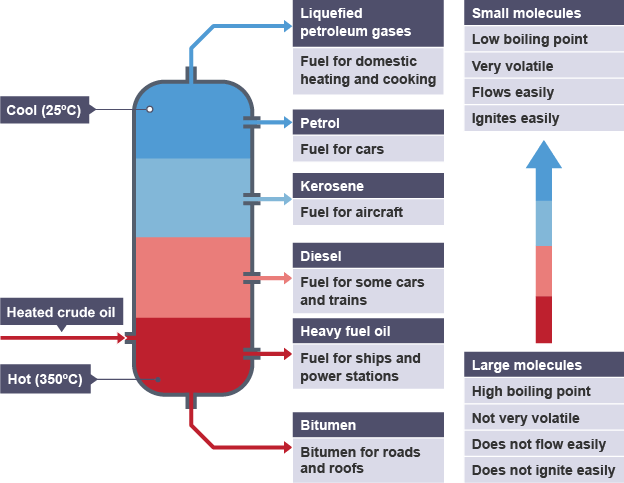
are smaller hydrocarbon chains more or less flammable and volatile than longer ones?
more flammable
more volatile
lower boiling point (will condense higher up the fractionating column)
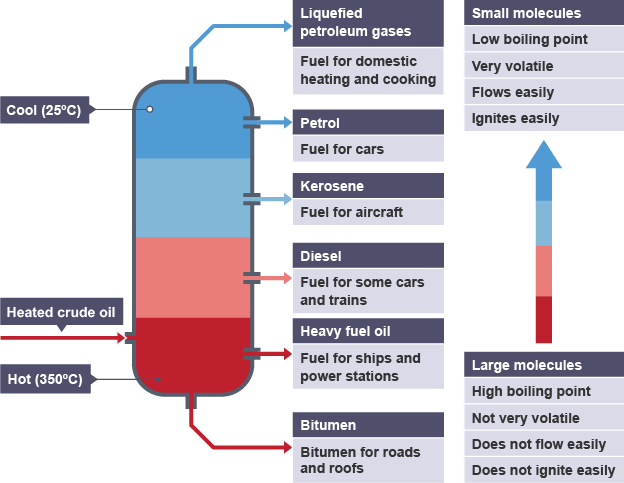
what are smaller hydrocarbon molecules most useful for?
fuels, as they are very flammable
what are large hydrocarbon molecules not useful for, and what can they be used for instead?
not useful for fuels (not as flammable as smaller)
either used for another purpose
or broken down into smaller hydrocarbons, via cracking
what are petrochemicals?
A petrochemical is a substance made from crude oil, via chemical reactions
eg. polymers, solvents, lubricants, detergents etc.
what is a feedstock?
a raw material used to provide reactants for an industrial reaction
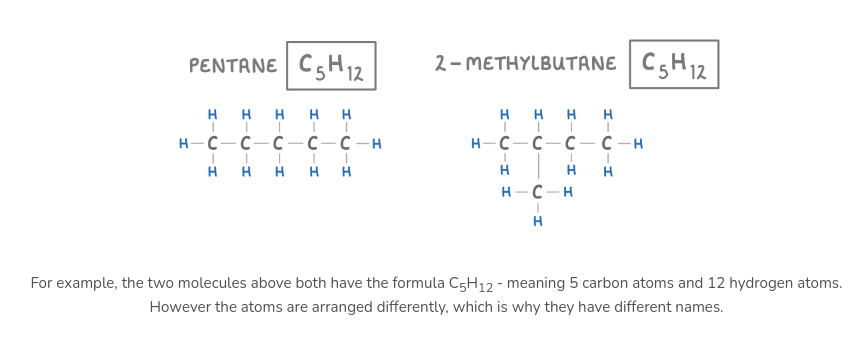
what is are isomers?
molecules with the same molecular formulas but different structural formulas
made of the same atoms, but arranged differently
what is cracking?
breaking down long hydrocarbons into shorter, more flammable (so more useful) hydrocarbons
this is a thermal decomposition reaction
what are the two types of cracking?
catalytic cracking
steam cracking
what are the steps for catalytic cracking?
heat (and vapourise) the long-chain hydrocarbon
pass hydrocarbon vapour over hot, powdered aluminium oxide (the catalyst)
as the long hydrocarbons come into contact with the catalyst, they split apart into two, smaller hydrocarbons
what are the steps for steam cracking?
heat (and vapourise) the long-chain hydrocarbon
mix with steam at very high temperatures
this causes the long hydrocarbon chains to split apart into two smaller ones
what is the word equation for cracking hydrocarbons?
long chain alkane → shorter alkane + alkene
eg. decane → heptane and propene
the number of hydrogens and carbons on both sides must be the same (balanced)
are alkenes saturated or unsaturated?
unsaturated
which are more reactive: alkanes or alkenes?
alkenes
what is the test for alkenes?
the bromine water test
if added to a solution containing alkenes, bromine water will turn from orange to colourless
this is because alkenes are very reactive so react with the bromine water (this would not happen with alkanes, and the water would remain orange)
what is the catalyst used in catalytic cracking of hydrocarbons?
hot, powdered aluminium oxide
what does an electrochemical cell do?
converts energy between chemical and electrical forms
eg. fuel cells
what does a hydrogen-oxygen fuel cell do?
converts the chemical energy of hydrogen fuel and oxygen into electrical energy
combines hydrogen and oxygen to form water, whilst creating lots of energy
what does a hydrogen-oxygen fuel cell look like?
central electrolyte (typically KOH - potassium hydroxide)
electrodes either side, connected by a wire
electrodes are made of porous carbon, and also contain a catalyst to speed up the reaction
anode and cathode compartments, with inlets (for hydrogen at anode and oxygen at cathode)
outlet for water and heat at the cathode compartment
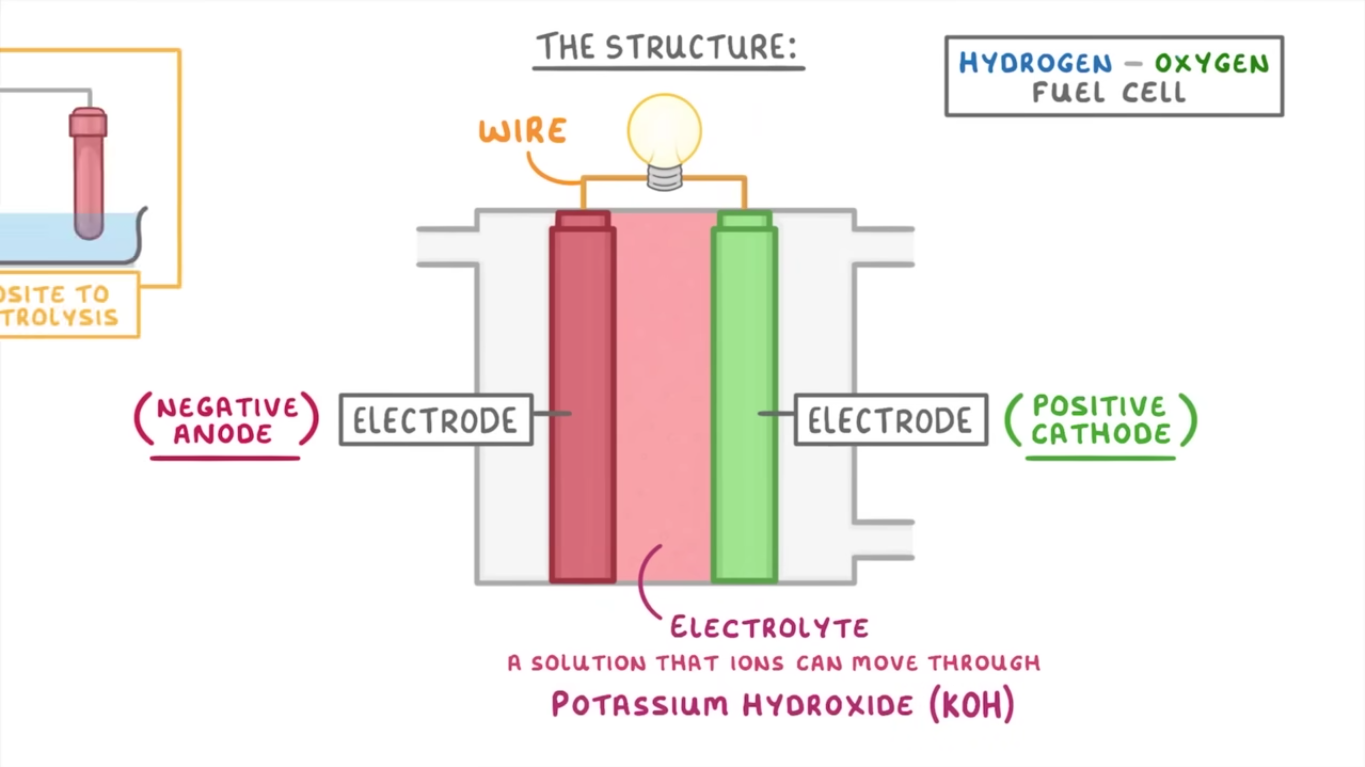
how does a hydrogen-oxygen fuel cell work?
hydrogen enters and is oxidised by the anode
H2 → 2H+ + 2e-
electrons pass along the wire to the cathode, and hydrogen ions move through the electrolyte to the cathode
electrons and hydrogen ions react with incoming oxygen at the cathode, forming water
O2 + 4H+ + 4e-→ 2H2O
water leaves the fuel cell via the outlet
how is electrical energy generated in a fuel cell?
as the fuel enters the cell, it becomes oxidised
this sets up a potential difference across the cell
this causes electrons to move through the wire, across the circuit, generating electricity
what could hydrogen-oxygen fuel cells be used to replace in the future?
replacing fossil fuel engines and batteries in vehicles (polluting)
hydrogen-oxygen fuel cells don’t produce any pollutants as waste products, last longer than batteries and are less polluting to dispose of
what are the pros of hydrogen-oxygen fuel cells?
only require hydrogen and oxygen (both renewable, no fossil fuels)
don’t produce carbon dioxide or other pollutants as waste products
relatively simple devices
last longer than batteries
are less polluting to dispose of
what are the cons of hydrogen-oxygen fuel cells?
hydrogen is gas - takes more storage space than fossil fuels or batteries
hydrogen is explosive when mixed with air - dangerous to store
making hydrogen fuel requires energy (often from fossil fuels)