3. Excitatory Amino Acid Neurotransmitters
1/77
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
78 Terms
Excitatory amino acid neurotransmitters examples (3)
Glutamate, aspartate, homocysteic acid
what do excitatory amino acid neurotransmitters do
Produce an excitatory response in neurons
• i.e., the neuron is more likely to send an action potential
Most important NT for normal brain function
Glutamate (Nearly all excitatory neurons in CNS are glutamatergic)(but remember glutamate is present in all cells!)
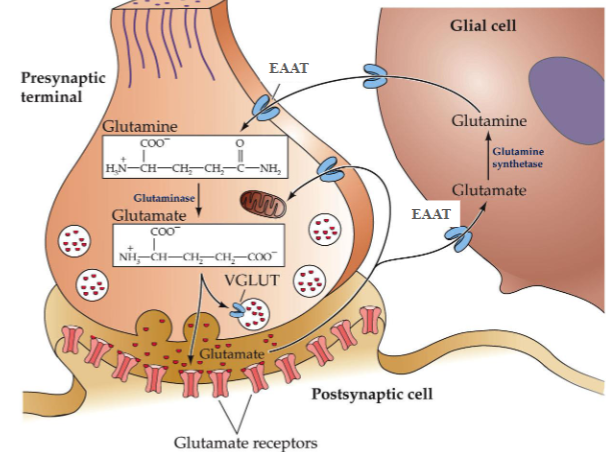
Glutamate is synthesised from ______ by what enzyme
Glutamate is synthesised from glutamine by glutaminase
Glutamate that has been released is taken up by what
astrocytes
When glutamate is taken up by astrocytes, it is converted to what by what enzyme
it is converted back to glutamine by glutamine synthetase
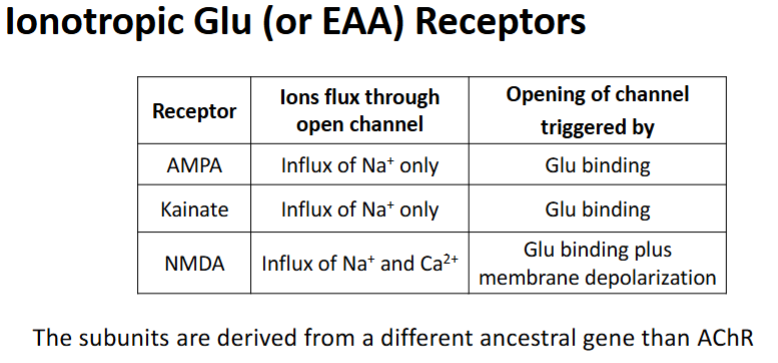
Antagonists of the NMDA receptor
D-AP5
MK801
Mg2+
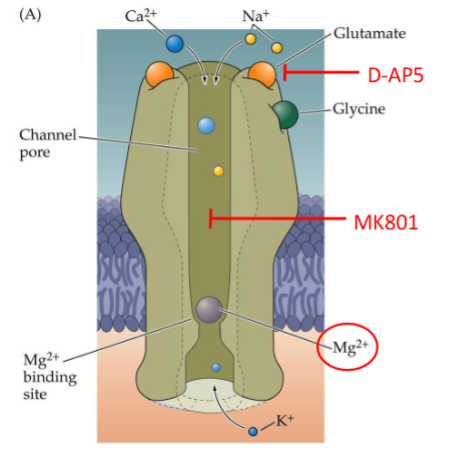
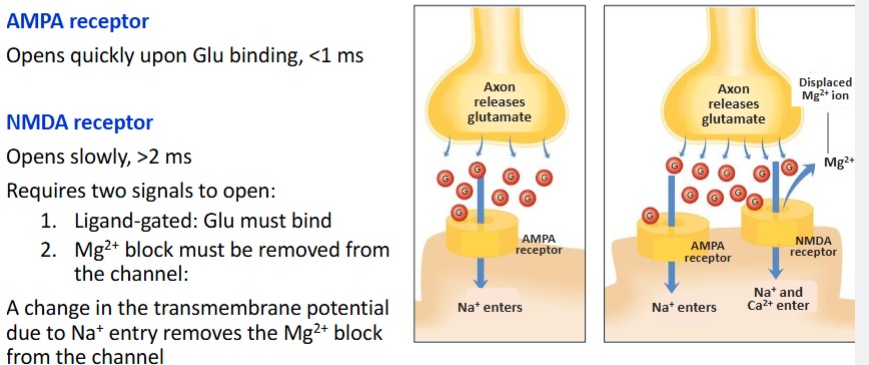
Significance of AMPA receptors & NMDA receptors being on the same membrane generally
The opening of NMDA receptor is reliant on the AMPA receptor
How is the NMDA receptor reliant on the AMPA receptor
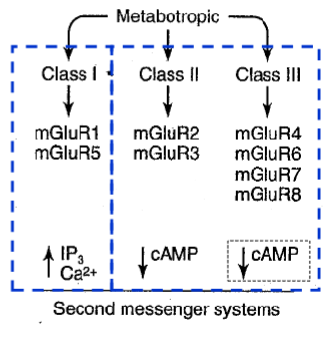
How many classes & subtypes of Metabotropic Glu receptors are there
3 classes: at least 8 subtypes, mGluR1-8
On what part of the synapse do metabotropic Glu Groups 1-3 act
Group I mainly postsynaptic
Group II and III - mainly presynaptic
Metabotropic Glu Group 1 is linked to the activation/inhibition of what
activation of phospholipase C
Metabotropic Glu Group 2 & 3 are linked to the activation/inhibition of what
Linked to inhibition of adenylate cyclase
What Metabotropic Glu receptor subtypes fall under what groups
Metabotropic Glu receptors may also play a role in memory formation - how?
NMDA receptor activation potentiates signalling via mGluRs
mGluR inhibitors block memory formation at some synapses
Link between excitatory amino acid receptors & stroke
It is usually excitatory amino acid receptors that cause the damage associated with strokes
How do excitatory amino acid receptors cause the damage associated with strokes
With the ischemia that comes with the block in blood supply, there is excessive Glu release → causes over-stimulation of NMDA receptors → Excess Ca2+ influx into postsynaptic neurons → Leading to excitotoxic cell death (excitotoxicity)
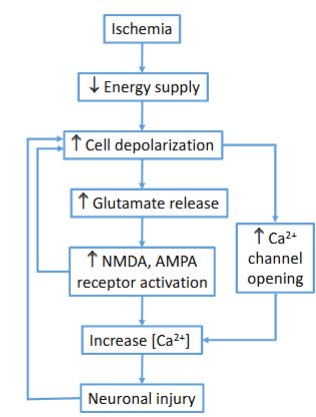
Explain how the bold part works: With the ischemia that comes with the block in blood supply, there is excessive Glu release → causes over-stimulation of NMDA receptors → Excess Ca2+ influx into postsynaptic neurons → Leading to excitotoxic cell death (excitotoxicity)
Large influx of Ca2+…
Can activate proteases (calpains), phospholipases, nitric oxide synthase (NOS), nucleases etc. → Leads to very rapid cell death by necrosis
Is spotted & gathered into mitochondria resulting in swelling and eventual rupture of mitochondria → Leads to delayed cell death
NMDA-R antagonists; _____ and _____ provide protection in models of ischemia
NMDA-R antagonists D-AP5 and MK801 provide protection in models of ischemia
Take a moment to compartmentalise :)
Give an example of an inhibitory amino acid neurotransmitter
GABA
What do inhibitory amino acid neurotransmitters do
They cause an influx of negatively charged ions such as Cl-
Inside of cell becomes more negative
Hyperpolarisation of post-synaptic cell making it less likely to initiate an action potential
What is the main inhibitory NT in the brain
γ-aminobutyric acid (GABA)
Approx ___% of synapses in brain uses GABA
Approx 33% of synapses in brain uses GABA
Presence of ____ indicates a GABAergic neuron
Presence of GAD indicates a GABAergic neuron
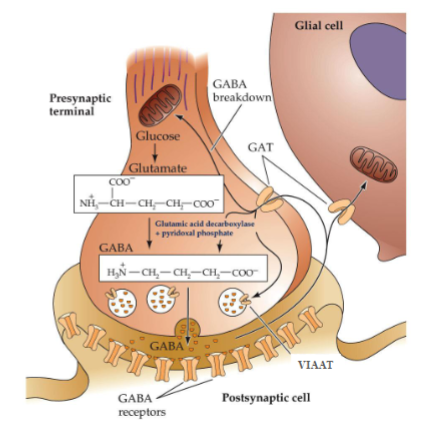
From which amino acid is GABA synthesized
Glutamate.
Which enzyme converts glutamate into GABA
With which cofactor does it do this?
Glutamic acid decarboxylase (GAD), with pyridoxal phosphate as a cofactor.
Which transporter loads GABA into synaptic vesicles?
VIAAT (Vesicular Inhibitory Amino Acid Transporter).
What happens to GABA when the presynaptic terminal is depolarized?
GABA-filled vesicles fuse with the membrane and release GABA into the synaptic cleft.
Which transporter clears GABA from the synaptic cleft?
GAT (GABA transporter).
Where is GABA taken after reuptake?
Into presynaptic neurons or glial cells.
True/False GABA receptors can be Ionotropic & Metabotropic
True
Ionotropic: GABAA and GABAC
Metabotropic: GABAB
The GABAA receptor is structurally related to which receptor?
The nicotinic acetylcholine receptor (nAChR).
Which ion does the GABAA receptor channel selectively allow through?
Chloride (Cl⁻) ions.
How is the action of GABA at the GABAA receptor terminated?
GABA is removed from the synaptic cleft by GABA transporters (GAT).
Effect of Benzodiazepines on the GABAA receptor
They increase the frequency of Cl⁻ channel opening when GABA is present → Increased hyperpolarisation → increased relaxant
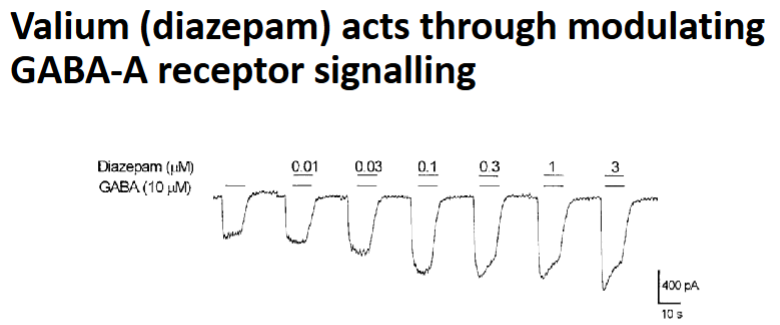
Effect of Barbiturates on the GABAA receptor
They increase the duration of Cl⁻ channel opening when GABA binds.
Can directly activate the receptor even without GABA
Increase hyperpolarisation
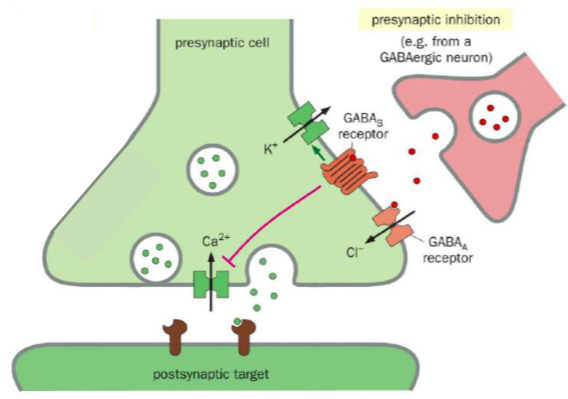
How does Presynaptic inhibition work
Inhibitory NT binds to receptors on the presynaptic cell →
Reduction in depolarisation of the presynaptic nerve terminal → Less Ca2+ influx → Less excitatory NT release
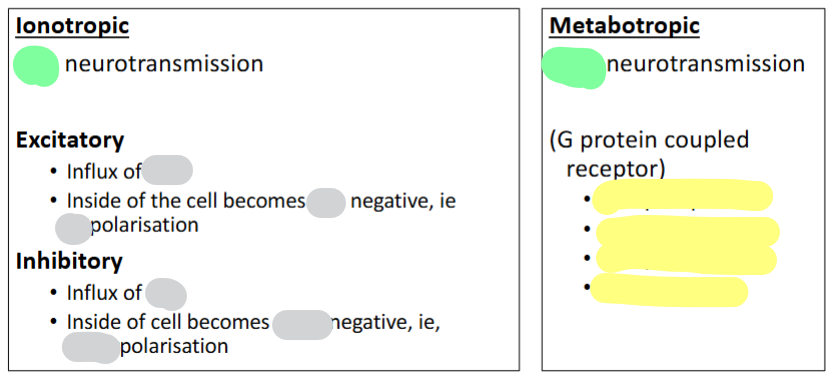
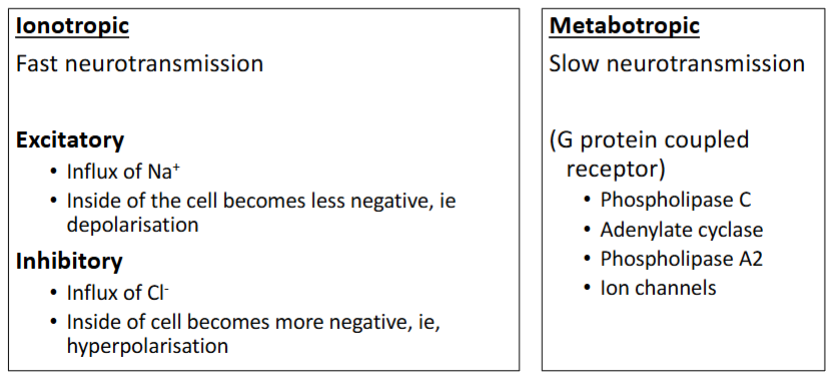
Grey: fill in blank
Green: Fast/Slow
Yellow: Give 4 examples
What are Biogenic amines
Bioactive amine neurotransmitters
What do Biogenic amines do
Implicated in wide range of behaviours
E.g., movement, reward, addiction, depression, sleep
What do Biogenic amines all have in common
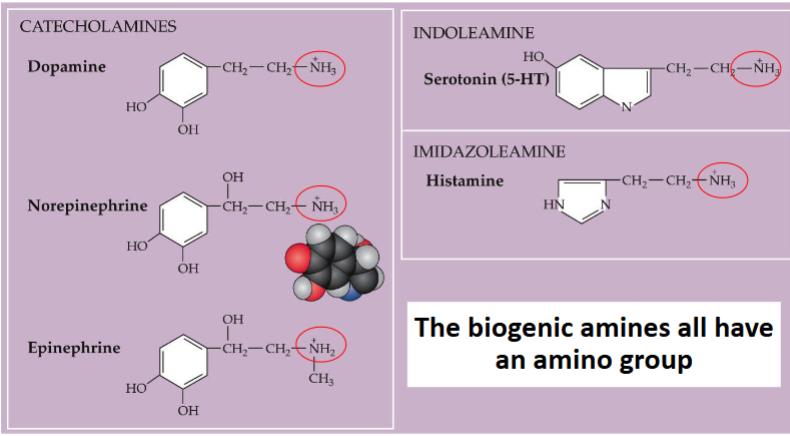
Catecholamines structure
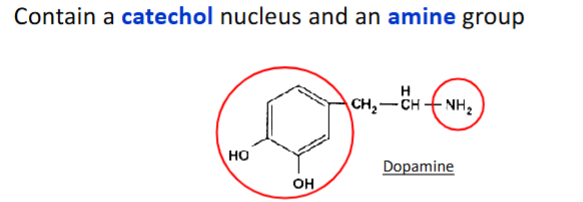
What catecholaminergic neuron is for the NT dopamine
Dopaminergic neurons
What catecholaminergic neuron is for the NT norepinephrine
Adrenergic neurons
What catecholaminergic neuron is for the NT epinephrine
Adrenergic neurons
catecholamine neurotransmitters are all derived from what
They are all derived from tyrosine
Explain the pathway from tyrosine to catecholamine neurotransmitters such as epinephrine
(no enzymes - just intermediates)
Tyrosine → DOPA → Dopamine → Norepinephrine → Epinephrine
The rate limiting step in the formation of catecholamine neurotransmitters is controlled by what enzyme
Tyrosine hydroxylase
Where is Tyrosine hydroxylase found
Only found in sympathetic neurons and adrenal chromaffin cells
If a cell has tyrosine hydroxylase, it is known as a what
catecholaminergic cell
Which transporter loads monoamines into synaptic vesicles?
Vesicular monoamine transporter (VMAT).
Which neurotransmitter classes does VMAT transport?
Catecholamines (dopamine, norepinephrine, epinephrine) and indoleamines (e.g., serotonin).
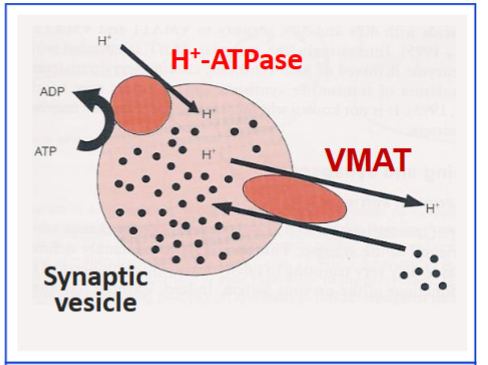
What drug inhibits VMAT?
Reserpine.
Which enzyme is found inside vesicles of adrenergic neurons?
Dopamine β-hydroxylase (DBH).
What is the function of dopamine β-hydroxylase?
It converts dopamine into norepinephrine inside vesicles.
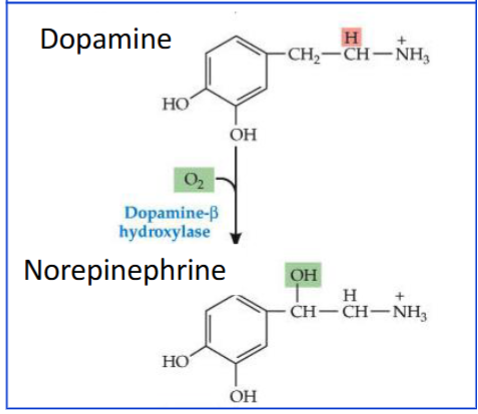
What are the vesicles in adrenal chromaffin cells called?
Chromaffin granules.
True/False Dopamine receptors, D1, D2 &D5 only are coupled to G proteins
False: Dopamine receptors, D1-D5 are all coupled to G proteins
There are 2 types of dopamine receptors D1-like receptors and D2-like receptors. What receptors (1-5) are in each of these groups & what do they do?
D1-like receptor: D1 and D5 – activate adenylate cyclase (increases cAMP)
D2-like receptor: D2,3,4 – inhibit adenylate cyclase (decreases cAMP)
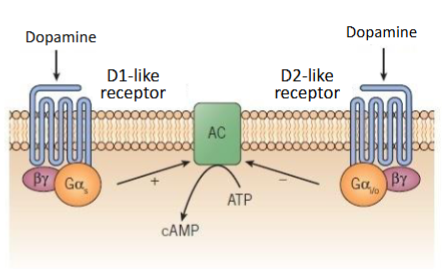
What is the main mechanism for inactivation of dopamine and other biogenic amines
Reuptake into presynaptic terminals or glial cells.
Which transporter is responsible for dopamine reuptake?
Na+-dependent DA (dopamine) transport protein
Which drugs inhibit dopamine reuptake via DAT (dopamine transporter)
Cocaine and amphetamines
Besides reuptake, how else can biogenic amines be inactivated?
By enzymatic degradation.
Minor way: Diffusion out of the synaptic cleft.
Which enzymes degrade dopamine
Monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT).
How does MAO: Monoamine oxidase degrade dopamine
MAO enzymes deaminate catecholamines → inactive derivatives
Where does MAO: Monoamine oxidase degrade dopamine
Associated with outer mitochondrial membrane
How does COMT: Catechol-O-methyltransferase degrade dopamine
Transfers methyl groups to hydroxyl group of catechols
Where does COMT: Catechol-O-methyltransferase degrade dopamine
Cytosol
Which enzyme MAO: Monoamine oxidase / COMT: Catechol-O-methyltransferase works with AD (aldehyde dehydrogenase)
MAO: Monoamine oxidase
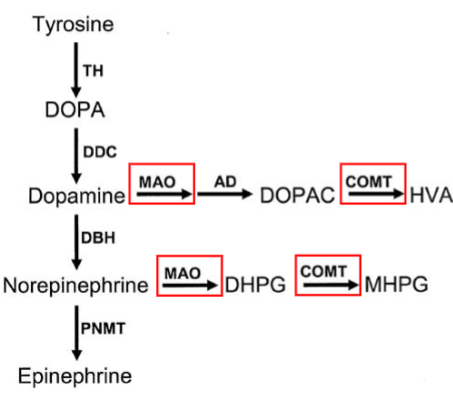
What diseases is dopamine associated with
• Parkinson's disease
• Attention deficit hyperactivity disorder
• Tourette syndrome
• Schizophrenia
• Bipolar disorder
• Addiction
What is Parkinson’s disease
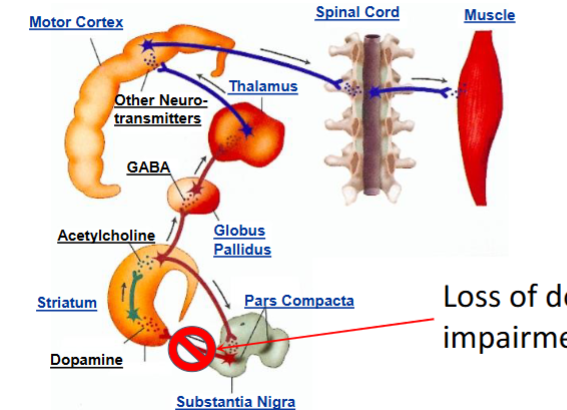
Insufficient dopamine in the nigrostriatal pathway
Dopaminergic neurons project from where to where
Dopaminergic neurons project from the substantia nigra to the striatum
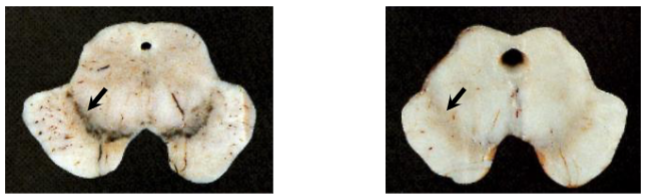
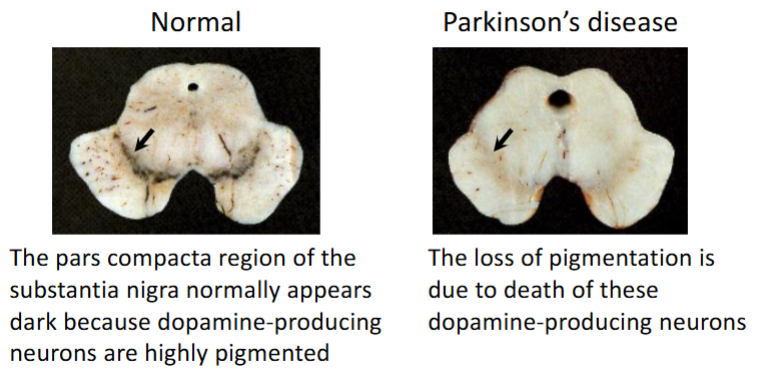
Which one has Parkinson’s disease? Explain
How does loss of dopamine cause impairment of motor control
Causes of Parkinson’s disease

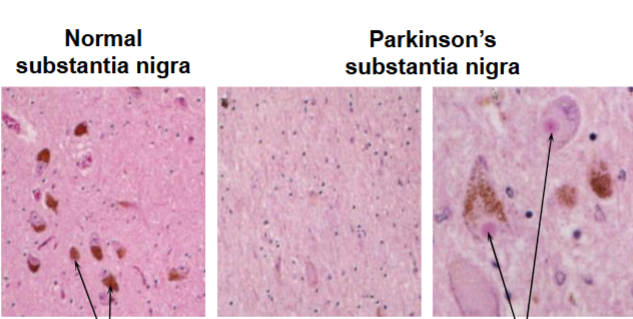
How would you know that the second 2 pictures have Parkinson’s disease
Loss of pigmented neurons
Presence of Lewy bodies
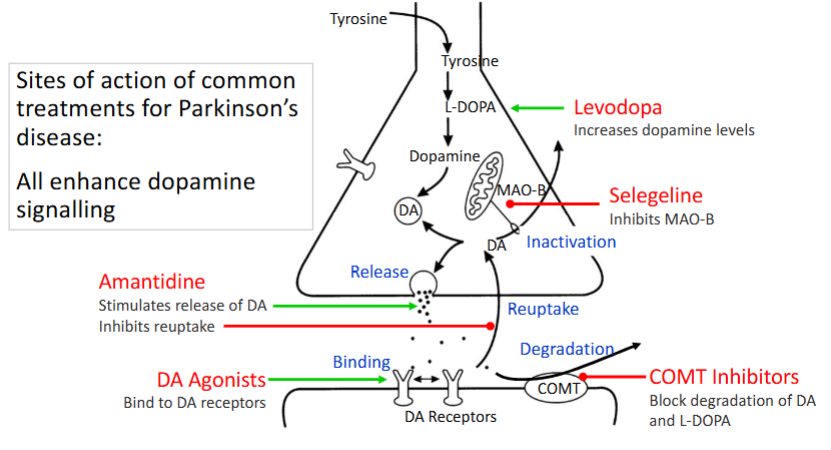
What are some treatments for Parkinson’s disease
Written in red