Topic 1: Inorganic Chemistry I - Introduction to Transition Metals
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
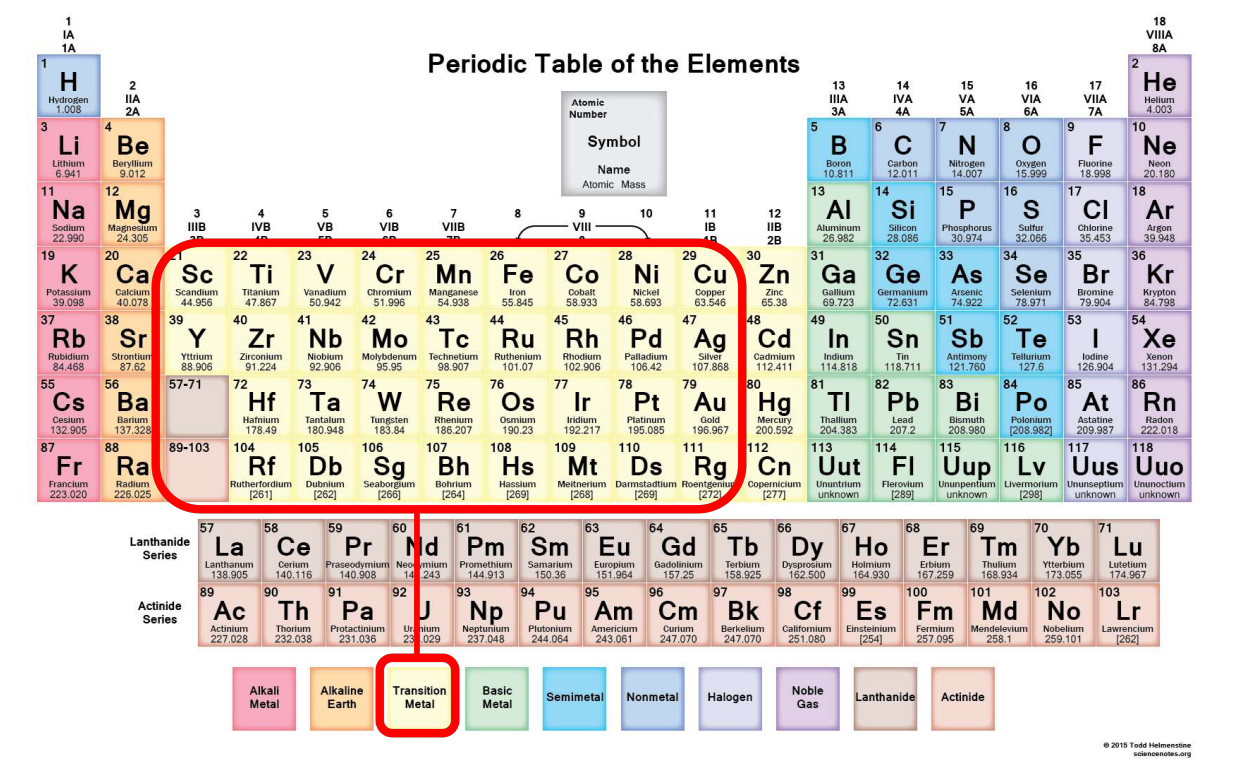
Transition Metals (TMs)
Transition metals are elements in groups 3 to 11 of the periodic table that have partially filled d-orbitals.
Criteria for TMs
Elements must have an incompletely filled d-shell to be considered transition metals.
TM Exclusions
Zinc (Zn), Cadmium (Cd), and Mercury (Hg) in the 12th group are not counted as TMs by IUPAC because they have full d-shells (d10) and no valence d-orbitals.
Rows of TMs
There are four rows of transition metal elements. The first row includes chromium, iron, nickel, copper, and zinc. The fourth row elements are man-made and mainly studied in experiments.
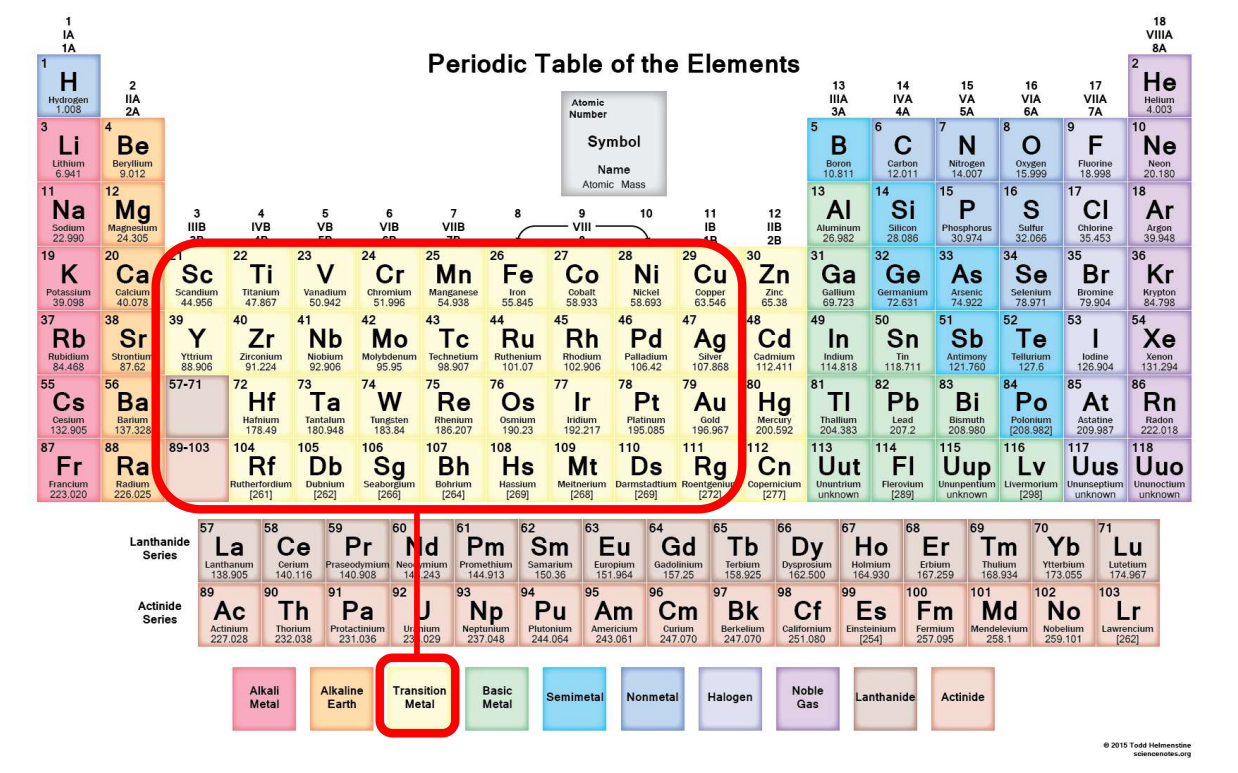
Bonding with d-orbitals in TMs
d-orbitals are involved in bonding, unlike in most carbon-based chemistry where only s and p-orbitals are involved.
Capacity of d-Shell
A d-shell consists of 5 orbitals and can hold up to 10 electrons.
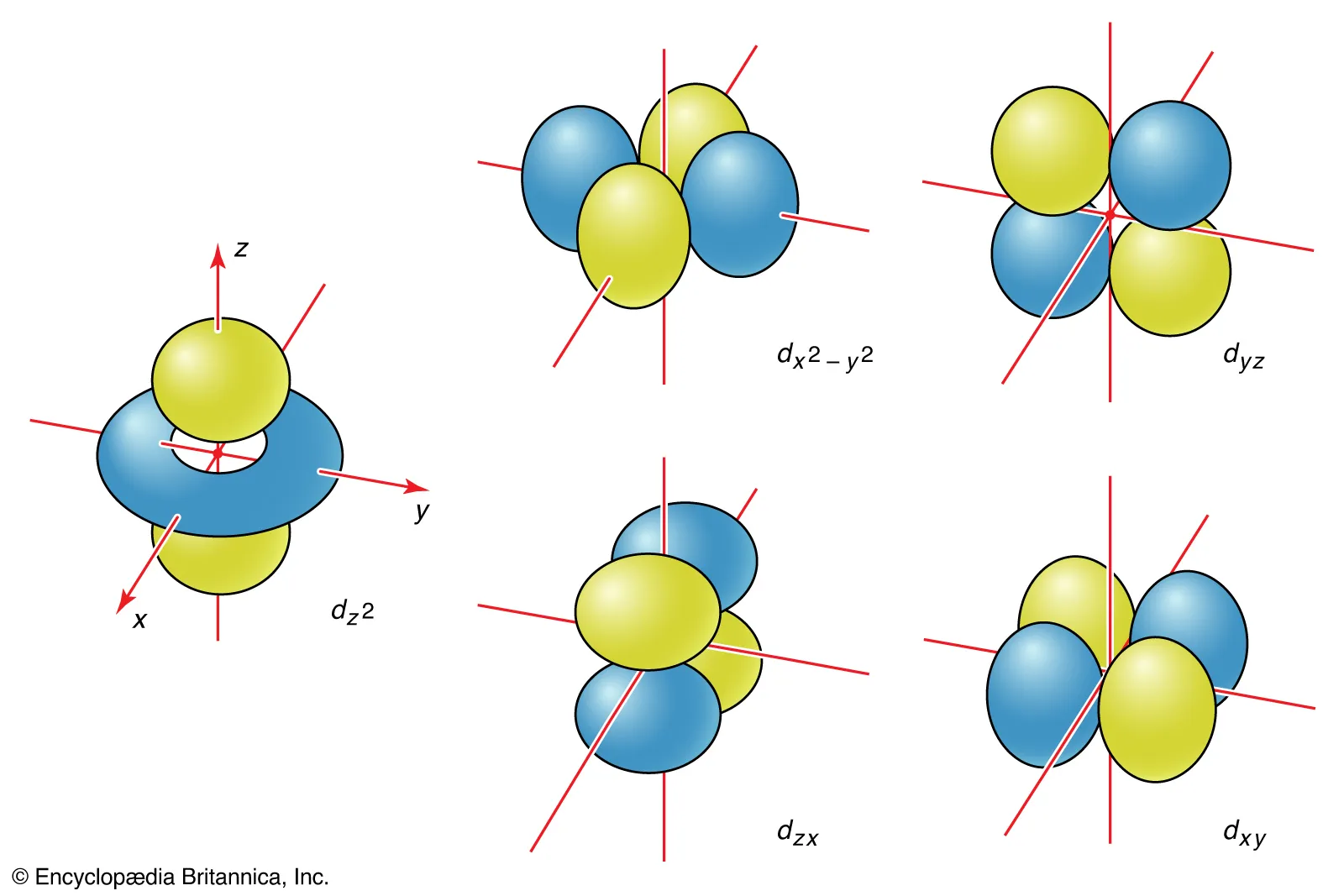
Nature of d-Orbitals
They are five degenerate orbitals (l = 2, ml = -2, -1, 0, 1, 2) in free atoms or ions that are highly directional, and poorly shielded.
d-Orbital Labels
d-orbitals are conventionally labeled dxy, dxz, dyz, dx2-y2, and dz2.
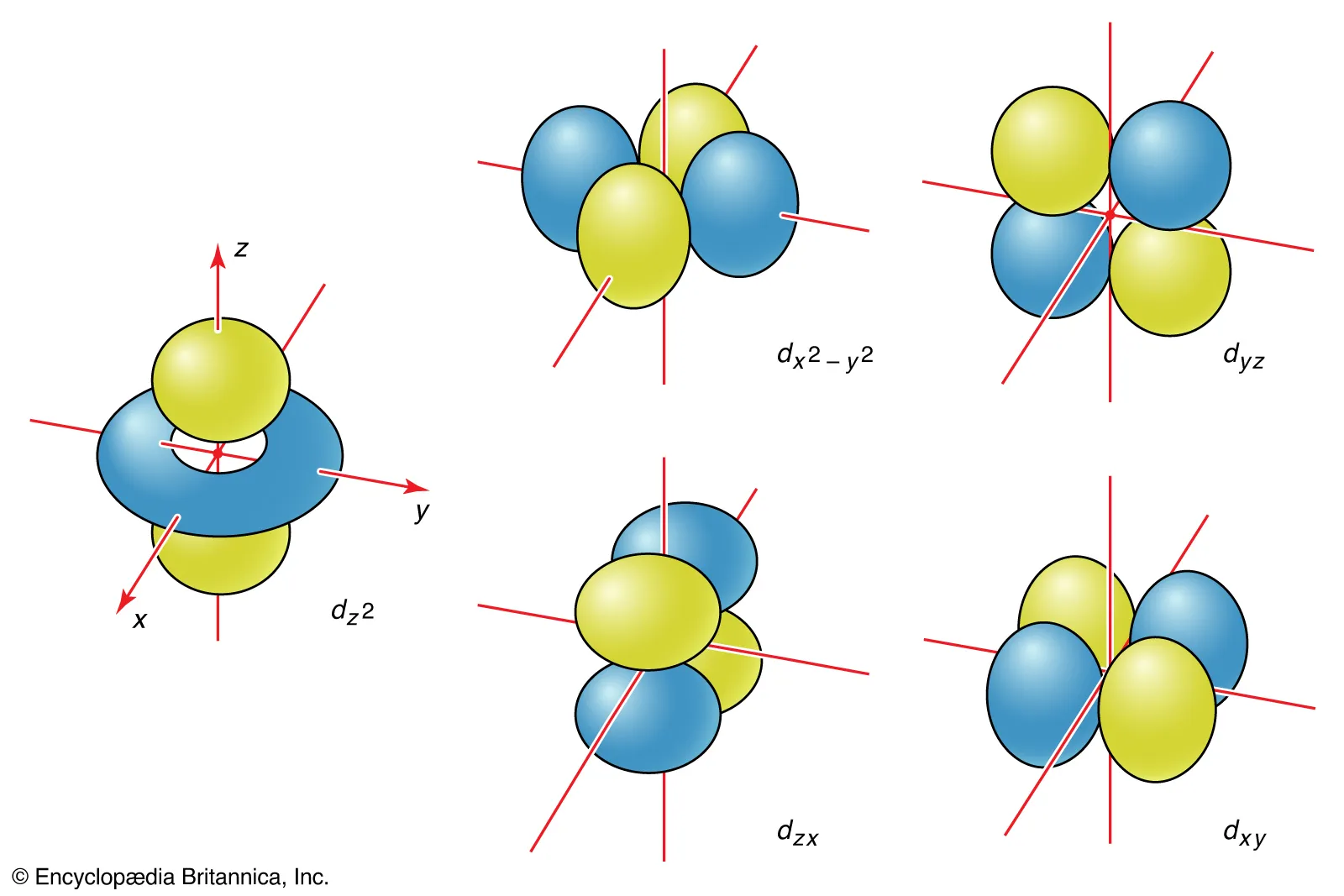
d-Orbital Labels: dxy, dxz, dyz
These orbitals have 4 lobes pointing in between the principal axes.
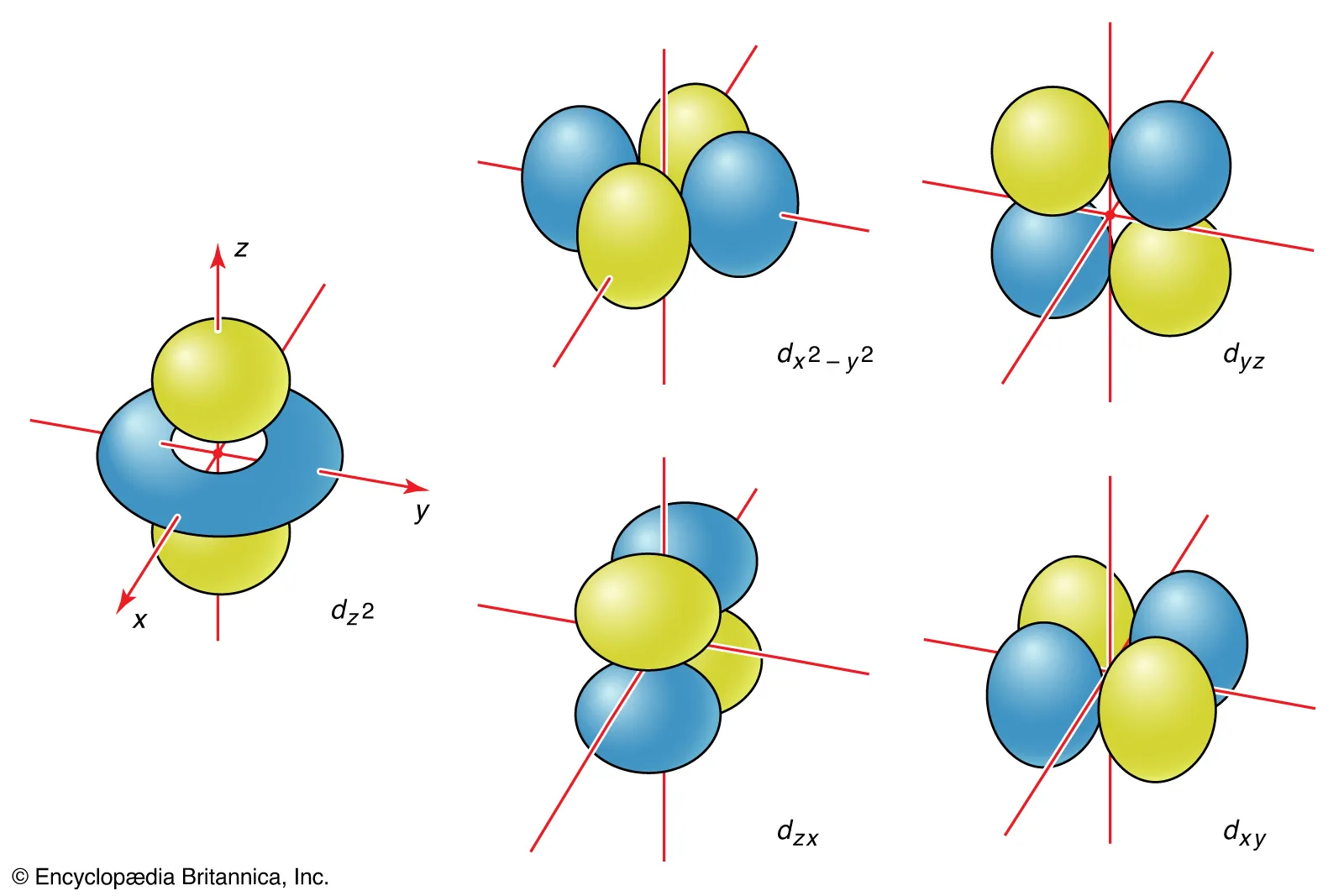
d-Orbital Labels: dx2-y2 and dz2
These orbitals have lobes pointing along the axes.
dz2 has a dumbbell along Z axis and a torus in the XY plane.
General Electronic Configuration of TMs
[Noble gas] ns2 (n-1)dx, where:
n ranges from 4 to 6
x ranges from 1 to 10.
Filling Order of TMs
For neutral atoms, ns orbitals fill before (n-1)d orbitals: 4s before 3d, 5s before 4d, 6s before 5d.
Example:
Titanium (Ti) configuration is [Ar] 4s2 3d2.
Vanadium (V) configuration is [Ar] 4s2 3d3.
![<p>For neutral atoms, ns orbitals fill before (n-1)d orbitals: 4s before 3d, 5s before 4d, 6s before 5d.</p><ul><li><p>Example: </p><ul><li><p>Titanium (Ti) configuration is [Ar] 4s<sup>2</sup> 3d<sup>2</sup>.</p></li><li><p>Vanadium (V) configuration is [Ar] 4s<sup>2</sup> 3d<sup>3</sup>.</p></li></ul></li></ul><p></p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/08f8b3bb-61bf-4c49-a031-4554ff8c6429.png)
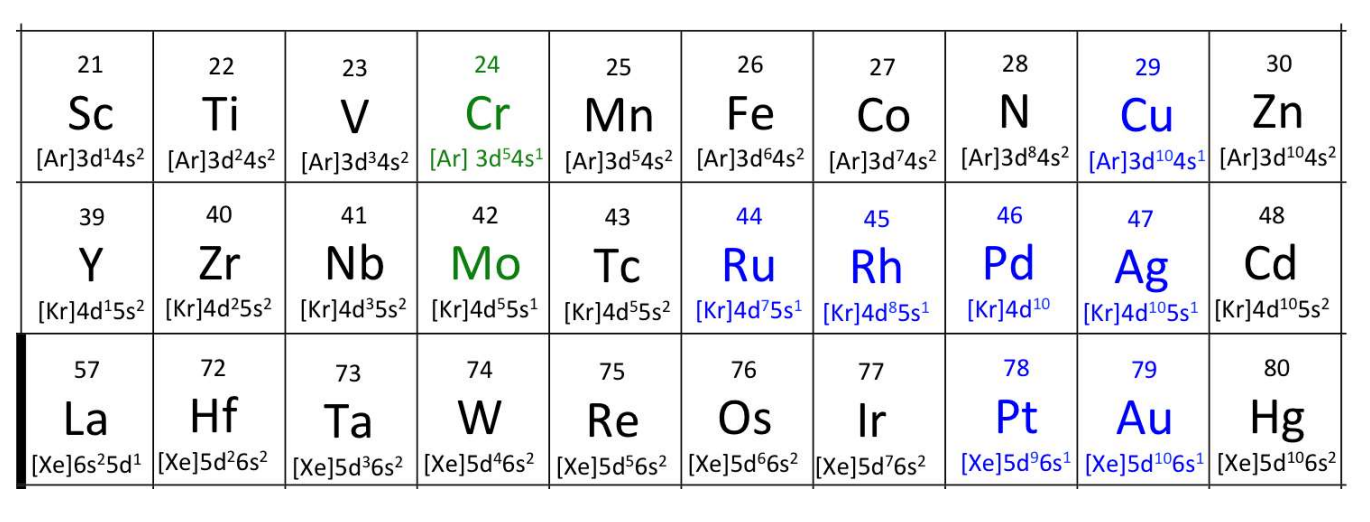
Filling Order of TMs: Exceptions
Half-filled (d5) or fully filled (d10) subshells are extra stable and alter the filling order.
Example:
Chromium (Cr) is [Ar] 4s1 3d5 instead of [Ar] 4s2 3d4.
Copper (Cu) is [Ar] 4s1 3d10 instead of [Ar] 4s2 3d9.
![<p>Half-filled (d<sup>5</sup>) or fully filled (d<sup>10</sup>) subshells are extra stable and alter the filling order.</p><ul><li><p>Example:</p><ul><li><p>Chromium (Cr) is [Ar] 4s<sup>1</sup> 3d<sup>5</sup> instead of [Ar] 4s<sup>2</sup> 3d<sup>4</sup>.</p></li><li><p>Copper (Cu) is [Ar] 4s<sup>1</sup> 3d<sup>10</sup> instead of [Ar] 4s<sup>2</sup> 3d<sup>9</sup>.</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/1dd590d0-2139-4ad7-b48e-ec2da6a96ddf.png)
5th and 6th Period TMs
Electronic configurations differ due to relativistic effects which become significant in heavier atoms → he high nuclear charge (Z) in heavy atoms causes → inner-shell electrons (and electrons with significant probability density close to the nucleus, like s electrons) to move at speeds approaching the speed of light
*Beyond scope of the course
Variable Oxidation States in TM Ions
Transition metal cations exhibit multiple oxidation states.
Left-side TMs commonly +3;
right-side +2;
middle elements (like Mn) range from +2 to +7.
TM Ionization Rule
When forming cations, ns electrons are removed before (n-1)d electrons, despite ns electrons being filled first as well
Electrons in Complexes
In complexes, as a result of the ionization rule, the 1st series TMs have remaining valence electrons in d-orbitals, not in 4s.
Example:
Vanadium(II) ion: Neutral V is 4s2 3d3, V2+ loses 4s electrons to become [Ar] 4s0 3d3.
d-Electron Count Formula
d-electron count = group number - oxidation number aka valence electrons - charge
Properties of TMs Due to d-Electrons
Transition metal complexes often show color, catalytic activity, and magnetic properties.
Properties of TMs Due to d-Electrons: Color
TM salts and complexes are often colored due to d-d transitions, while s- and p-block salts are usually colorless.
Examples: Pink rubies from Cr3+, blue sapphires from Ti3+.
d-d Transition Mechanism
The ligands’ negative charges repel the d-orbitals differently depending on their orientation → degeneracy removed → d-orbitals in the same sub-shell split into lower and higher energy groups → when d-orbitals are partially filled; photons are absorbed by electrons matching the d-d energy gap → electron jumps
Perceived Color after d-d Transition
While transitions with bigger gaps falls in the UV region (undetectable to the naked eye), d-d transitions have smaller gaps that fall in the visible light region.
The color we see is complementary to the light absorbed by d-d transitions.
Enzyme and Catalyst Function of TMs
TMs are critical in enzymes (biological catalysts) and industrial catalysts due to variable oxidation states.
Variable oxidation states allow for flexibility → better bioavailability
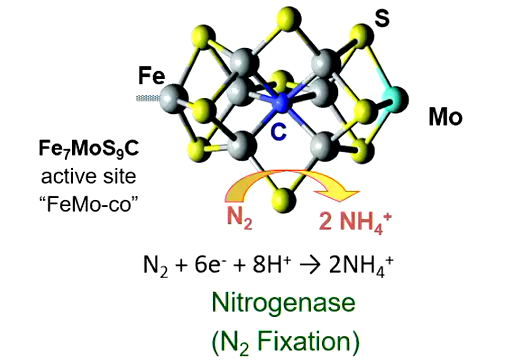
Enzyme and Catalyst Function of TMs: Nitrogenase
Enzyme that makes nitrogen more bioavailable
The N2 is triple bonded, requiring a lot of energy to separate
Nitrogenase cracks that apart and converts it: N2 + 6e- + 8H+ → 2NH4+
At the center is a cluster of metals: Fe7MoS9C
Having it in ammonium form makes it more water-soluble
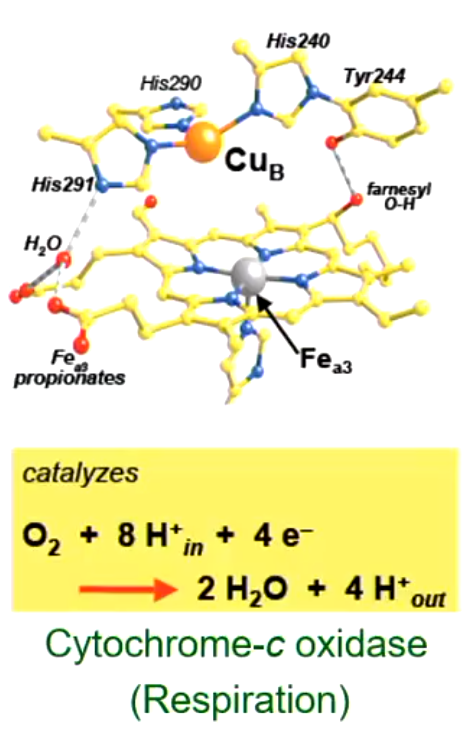
Enzyme and Catalyst Function of TMs: Cytochrome-C Oxidase
Enzyme that aids in respiration
Made up of coordination compounds with metals like iron and sulfur surrounded by ligands
It catalyzes: C2 + 8H+ + 4e- (in) → 2H2O + 4H+ (out)
Paramagnetic
Compounds with unpaired electrons, strongly attracted to magnetic fields.
Diamagnetic
Compounds with all paired electrons, weakly repelled by magnetic fields.
Magnetic Properties of TMs: Spin-Crossover
Compounds can switch between diamagnetic and paramagnetic with temperature changes.