Immunology Objectives (Final)
1/259
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
260 Terms
Describe innate and adaptive immunity and list their differences
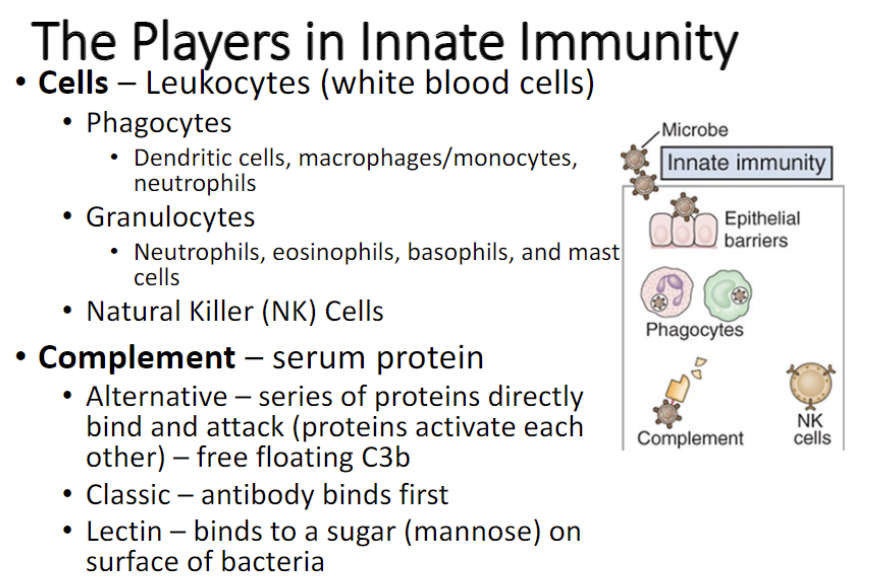
Innate immunity:
1st line of defense
Protects previously unexposed animals
Immediate protection, NOT specific
Activated by PAMPs & DAMPs (Pathogen-associated molecular patterns & damage-associated molecular patterns)
Provides signals for adaptive immunity
Adaptive immunity:
Develops days to weeks after exposure
Specific
Memory
Tolerance
Enhance innate immune response (uses way more receptors)
List major components of the innate and adaptive immunity.
Innate immunity:
Physical/chemical barriers:
skin
Mucous membranes
Normal microflora
Acid environment in stomach
Antimicrobial peptides
Phagocytic and sentinel cells:
Phagocytic: ingest and kill pathogens.
Ex. Neutrophils and macrophages
Sentinel cells: resident tissue cells that detect invasion by recognizing PAMPs and DAMPs.
Ex. Dendritic cells (DC), macrophages, mast cells
Complement system
Series of 20-30 proteins in blood plasma
Rapidly induced
Multiple mechanisms for controlling infection
Potent; harmful if not regulated
An enzyme cascade system that has antimicrobial activity
Innate defense cytokines
Protein messenger molecules that can act on other cells or the cell that produced in helps regulate an immune response
Natural Killer (NK) cells
Lymphocyte that is part of innate immunity
Kill virus infected cells and tumor cells
Recognize and kill cells that do not express normal proteins
Adaptive defense:
Humoral immunity
(antibodies)
Cell-mediated immunity:
T- helper cells
cytotoxic T cells
Gamma delta (γδ) T cells
Describe the two main arms of the adaptive response.
Humoral immunity:
Named so because transfer of body ‘humors’ from protected animal to naive animal could provide protection
Mainly directed against bacterial invaders and is mediated by antibodies
B cells are a part of humoral/adaptive immunity.
Cell-mediated immunity:
Named so because transfer of cells from protected animal to naive animal could provide protection
Regulates innate and adaptive responses through cytokines and employs cells that destroy abnormal cells such as those infected by viruses
T cells are a part of cell-mediated/adaptive immunity
Define antigen, antibody and cytokines.
Antigen
A toxin or other foreign substance that induces an immune response in the body, especially the production of antibodies
Antibody
A blood protein produced in response to and counteracting a specific antigen
Cytokines
Protein messenger molecules that can act on other cells or the cell that produced it helps regulate immune response
Briefly explain memory and tolerance as it pertains to the adaptive immunity.
Tolerance means that immune cells do not respond to self-antigens (host proteins) so that it doesn't cause damage to the host itself. A healthy immune system should respond to foreign antigens but not self-antigens.
List types of white blood cells, approximate percentages of each, and their half-lives
Basophil
0.5% of WBC in circulation. Half life is 1-2 days
Contains granules (which stain basophilic) filled with inflammatory mediators (histamine, serotonin, etc.)
Secondary Importance in allergy and parasitic infections to eosinophil.
May or may not become tissue mast cells
Eosinophil
1-3% of WBC in circulation; half life is 30 minutes
Contains granules (stain eosinophilic) filled with potent mediators (major basic protein and eosinophilic cationic protein) capable of killing parasites
In blood stream for 30 minutes, then go to tissues and are found under epithelial surfaces; live weeks in tissue and then replaced by new cells
Important in control of extracellular parasites
Eosinophilia can occur in some parasitic infections and allergies
Monocyte
3-7% in circulation; circulate 1-2 days then migrate to tissue and differentiate into a macrophage
Macrophages are found in most tissues and are extremely important in immune response
Phagocytosis and killing of bacteria
Presentation of antigen on MCH II
Secretion of cytokines-major role in inflammation and immune response
Monocytes/macrophages arrive at the site of infection after neutrophils. Their accumulation at the site of inflammation is a sign of chronic infection
Neutrophil
55-90% of WBC in circulation
Short-lived, survive about 1-2 days at most
Half life in blood is 8-10 hours;approx 2.5 times a day all the neutrophils are replaced by new ones.
First responders to bacterial infections. Arrive within 4 hours. Exit at site of infection and accumulate in large numbers to ingest and kill pathogens
Pus=dead neutrophils
Bone marrow spends a lot of energy making neutrophils
Lymphocyte
20-35% in most animals; half-life of 120 days
B cells, T cells and NK cells
Circulate for 4 months between blood and lymphoid tissues searching for antigens. UNIQUE as other WBCs once exit blood, stay in tissues.
Naive B and T cells are the same morphologically. And are a part of adaptive immunity
If they do not come into contact with their antigen, they die.
If they meet their antigen, they get activated and some differentiate into memory cells.
Explain role of endothelial cells in immune responses.
Endothelial cells line blood and lymph vessels
Important for regulating leukocytic traffic
Have adhesion molecules, called addressins, that allow circulating leukocyte to know where they are located in the body.
Addressins are upregulated during infection to facilitate binding of neutrophils to the endothelial cells and their exit to the site of infection.
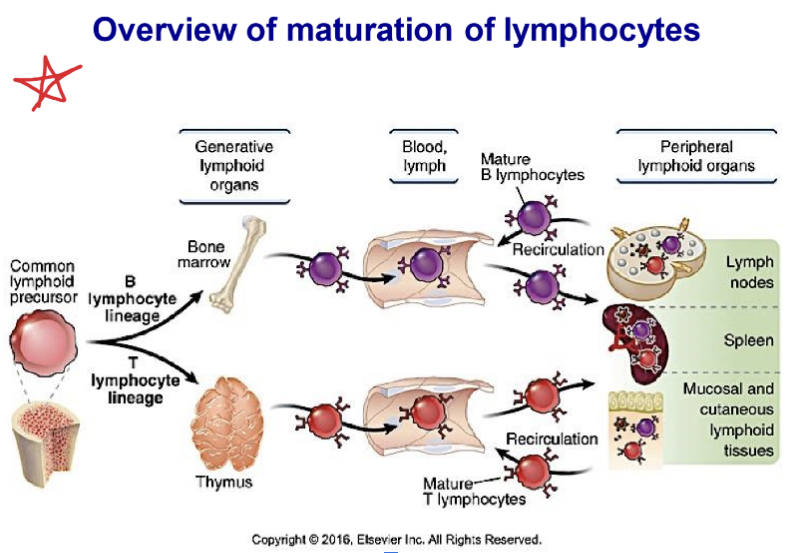
Know where different types of immune cells originate and mature
ALL IMMUNE CELLS ORIGINATE FROM BONE MARROW
ERYTHROID
RBCs, platelets
MYELOID
Monocytes, neutrophils, eosinophils, and basophils, some dendritic cells- important in initiation of immune response, mast cells
Granulocytes are released in mature state.
Dendritic cells migrate to tissues to mature.
These are important sentinel cells and antigen presenting cells. Key in initiation of adaptive immunity
Mast cell precursors leave bone marrow and mature in tissues. Live from weeks to months and are important in parasitic infection and allergies.
LYMPHOID
B cells, T cells, NK cells, some dendritic cells
T lymphocytes are released immature from the bone marrow as pre- T cells, which go to the Thymus to mature
B lymphocytes are released immature from the bone marrow as pre-B cells, which mature in primary lymphoid tissue:
Birds: mature in Bursa of Fabricius
Mammals: Mature in bone marrow (many species)
Peyer’s patch (part of GALT) at ileocecum in some species (ruminants)
NK cells are released mature from bone marrow.
They are NOT specific, unlike B and T cells.
Have NO memory and are a part of innate immunity.
List primary and secondary lymphoid tissues.
Primary lymphoid tissue: Maturation of lymphocytes take place here. They go from bone marrow and travel to tissues to mature.
In primary, sites of lymphocyte development
For T cells:
Thymus
For B cells:
Birds: Bursa
Primates, rabbits, rodents: Bone marrow
Ruminants, pigs, dogs: Peyer’s patch
Bone marrow
Secondary, sites where lymphocytes respond to antigens. Large number of lymphocytes reside here waiting for their antigen. SLT increases chances of antigen meeting.
Tonsils
Spleen:
Site for adaptive immune response to blood-borne antigens
Lymph nodes
Lymphocyte rich tissue connected to lymphatic system, where adaptive immune response to lymph-borne antigen is initiated.
MALT:
Mucosal associated lymphoid tissue, where adaptive immune response to antigens invading from the mucosal surfaces is initiated.
Peyer’s patches
Bone marrow
Describe distribution of lymphocytes in body
Lymph nodes: 40%
Bone marrrow: 10%
Intestine:10%
Spleen: 13%
Blood:2%
Other tissues:25%
DOES NOT STAY IN THE BLOOD
Explain overall lymphatic circulation
T cells circulate in both the bloodstream and the lymphatic fluid. Their precise route through a lymph node depends on whether they are naive or primed. Thus, naive lymphocytes enter lymph nodes through blood stream and high endothelial venues. Primed lymphocytes, in contrast, migrate through the tissues and enter through afferent lymphatics. They all leave through efferent lymphatics
.List three types of sentinel cells and describe their function.
Mast cell
Roles in inflammation, goes to hypothalamus, effects include: Fever, anorexia, sleepiness, depression
Macrophages
Goes to liver, and effects include: Increased synthesis of actue-phase proteins, iron, sequestration
Dendritic cells
HMGB-1 *acts on nearest blood vessels to allow WBCs to rush to injury. Effects include: Increased WBC production
Explain mechanisms of pathogen recognition by sentinel cells.
Cells have Pattern Recognition Receptors (PRRs) that recognize these alarm signals.
Signals are generated by invading microorganisms (exogenous signals) -PAMPs,
and dead or dying host cells (endogenous signals)- DAMPs, or alarmins.
Define and list examples of PRRs, PAMPs and DAMPs.
Examples of PAMPs:
Bacterial lipopolysaccharides
Bacterial Peptidoglycans
Bacterial DNA
Viral nucleic acids
Examples of DAMPs both extracellular and intracellular
Extracellular
Hyaluronic acid
Heparin sulfate
Fibrinogen
Collagen-derived peptides
Fibronectin
Laminin
Elastin
Intracellular
HMGB1*** important for cell damage and disruption
Uris acid
Chromatin
Adenosine
Galectins
S100 proteins
Cathelicidins
Defensins
N-formyl peptides
Lactoferrin
Heat-shock proteins
Examples of Pattern recognition receptors (PRRs)
Soluble
Collectins
Ficolins
Complement
Pentraxins
Within vesicle
TLR 3,7,8,9 (toll like receptors)* most important PRR
Cytoplasmic
Rig-1 (retinoic acid inducible gene)
NOD-like (nucleotide binding oligomerization domain)
Peptidoglycan receptors
DNA receptors
Membrane bound
TLRs
Lectins
Mannose receptor
Dectins
Scavenger receptors
Integrins
Define Toll-like receptors (TLRs) and explain their roles in recognition of foreign invaders
Transmembrane glycoprotein receptors that are present on many different cell types including sentinel cells
TLRs play a critical role in microbial sensing by recognizing bacteria, fungi, and viruses.
Describe location and specificities of different TLRs.
Mammals possess 10-12 diff functional TLRs
Human and cattle have TLR1 to TLR10
Mice have TLR1 to TLR9 and TLR11 to TLR13
The cell surface TLRs (TLR1,2,4,5,6,11, 12) recognize bacterial and fungal proteins, lipoproteins and LPS
intracellular TLRs (3, 7, 8, 9, 10) recognize viral and bacterial nucleic acids
Explain roles of TLRs in development of diarrhea in German Shepherds.
IBD dogs have several single-nucleotide polymorphisms (SNPs) in the TLR4 and TLR5 genes, which suggests that their TLR4 and TLR5 have reduced ability to defend against bacterial invasion, resulting in predisposition to enteric infections.
Explain downstream immunological events as a result of PAMP-induced activation of a TLR
Pathogen binds to TLR and signal cascade begins;
Produces gene transcriptions (MAPK, NF8, IRF3) and gene transcription happens and cytokines are produced in response, all depends on the bacteria sensed.
Define inflammation and explain how inflammation helps in combating infection.
Inflammation plays three essential roles in combating infection:
1. Deliver additional effector molecules and cells to sites of infection to augment the killing of invading microbes by the front-line macrophages
2. Provide a physical barrier preventing the spread of infection
3. Promote the repair of injured tissue
The main purpose of inflammation is to focus the immune response to the site of infection or injury.
Describe 4 cardinal signs of inflammation
Redness: is due to increased blood flow to the area of injury
Swelling: (edema) is due to increased extravascular fluid and phagocyte infiltration to the damaged area
Heat: is due to increased blood flow and the action of pyrogens (fever inducing agents)
Pain: is caused by local tissue destruction and irritation of sensory nerve receptors
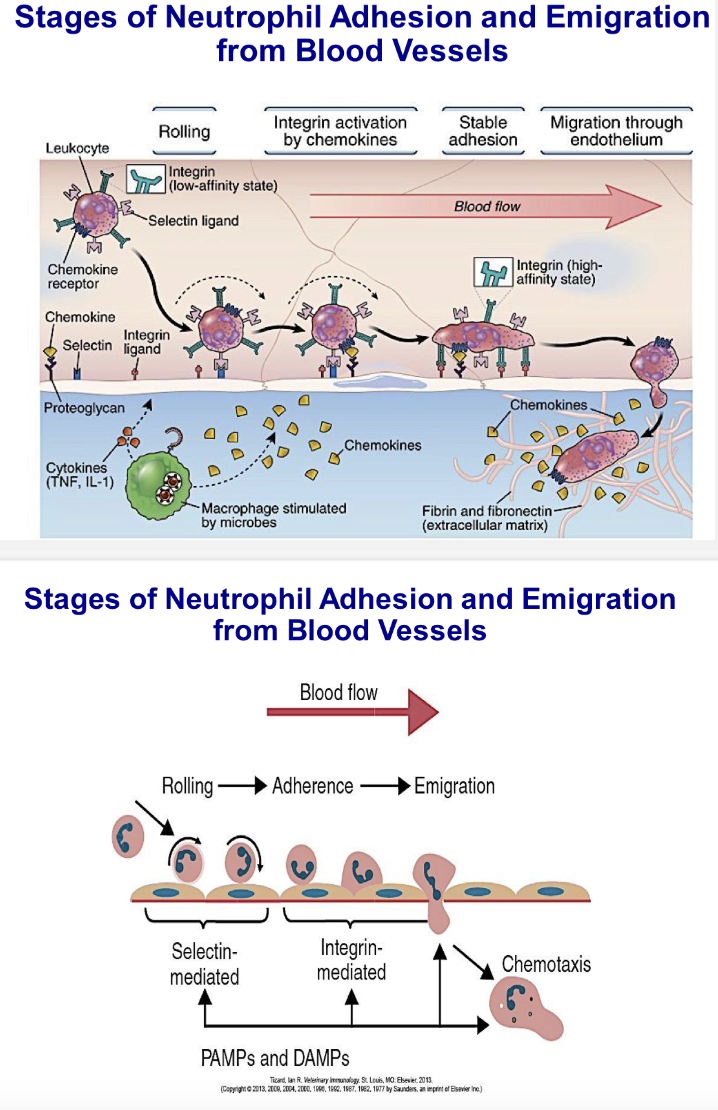
Describe stages of neutrophil adhesion and emigration from blood vessels and explain roles of different adhesion molecules in this process.
List different types of pro-inflammatory mediators.
Pro-inflammatory mediators:
Cytokines
Chemokines
Vasoactive amines
Vasoactive peptides
Vasoactive lipids
Coagulation system
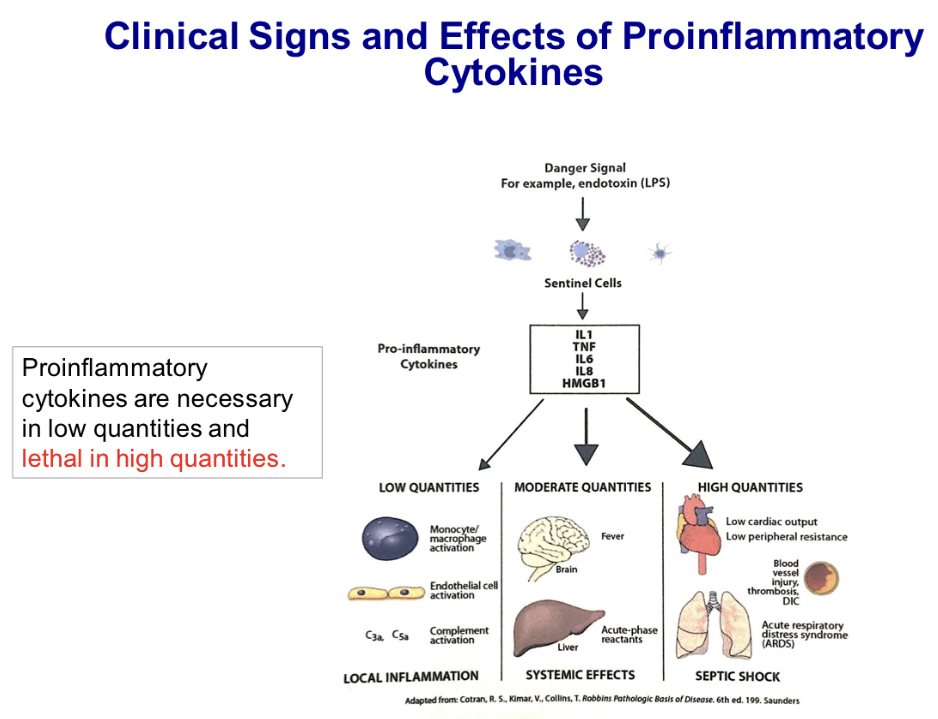
Describe clinical signs and effects of pro-inflammatory cytokines.
Depends on the quantity produced in response to PAMPs and DAMPs:
In low quantities, Local inflammation, which includes..
Macrophage activation, endothelium activation, complement activation
In moderate quantities, Local and system effects, including..
Fever, lethargy, loss of appetite from effects on hypothalamus, production of acute phase proteins from actions on the liver, and neutrophilia resulting from action on the bone marrow
In high quantities, Systemic vasodilation, increased vascular permeability
This leads to a drop in blood pressure and low cardiac output, vascular injury, thrombosis, and disseminated intravascular coagulopathy (DIC), pulmonary edema, air spaces fill with fluid, and leads to acute respiratory distress syndrome (ARDS)
Septic shock can occur in cows with Gram-negative bacterial mastitis. Endotoxin from bacteria induces high quantities of proinflammatory cytokines.
Cytokines and other mediators are produced by cells in response to microbial products and damaged host cells.
When exposed to infectious agents to their PAMPs, sentinel cells synthesize and secrete 3 major cytokines
Tumor necrosis factor-α (TNF-α)
Interleukin-1 (IL-1)
Interleukin-6 (IL-6)
These mediators increase the permeability of blood vessels, leading to the entry of plasma proteins into the tissues and promote the movement of leukocytes from blood into tissues.
Leukocytes destroy microbes, clear damaged cells and promote more inflammation and repair.
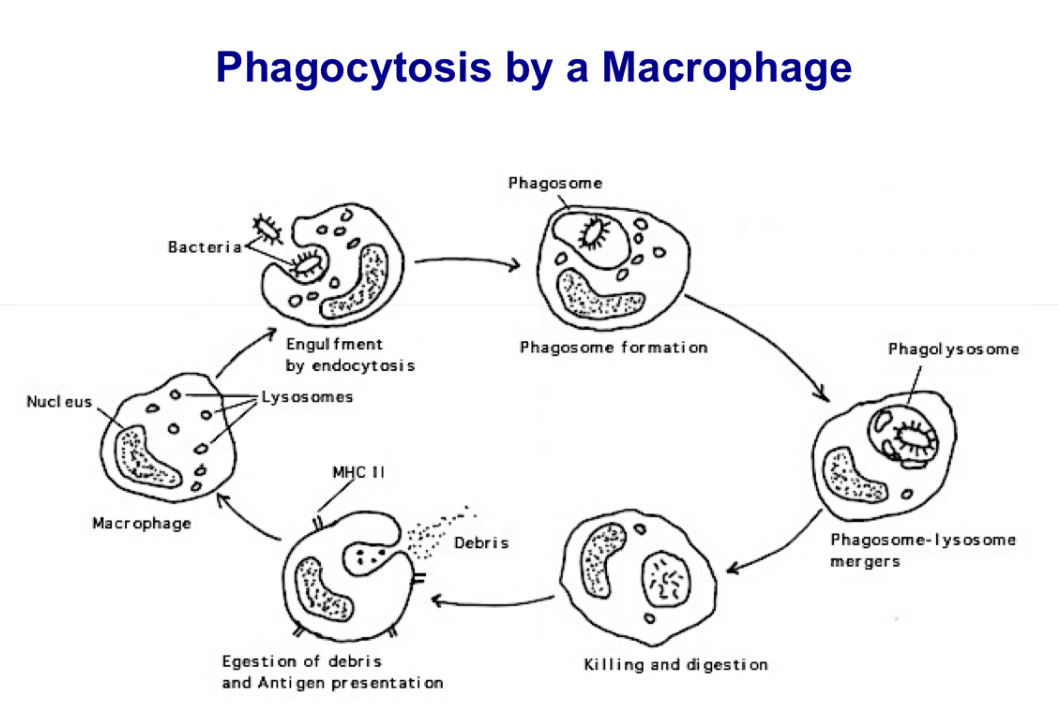
Describe phagocytosis and list professional phagocytes.
Phagocytosis: the ability of some cells to ingest foreign particles. “Eating by cells”
Phagocytes: Class of cells which are capable of ingestion and killing of microorganisms that incite inflammatory response. Neutrophils and macrophages are referred to as professional phagocytes for their role in the process. Neutrophils kill themselves in the process of killing bad cells; macrophages kill, strip bacteria or virus, then move on
List sequence of events in phagocytosis and describe each.
Phagocytosis and destruction of engulfed bacteria involves the following sequence of events:
Chemotaxis: Delivery of phagocytic cells to the site of infection
Adherence: Phagocytic adherence to the target
Ingestion: Engulfment of the target particle
Destruction: Intracellular killing and digestion of the target.
-Egestion only in case of macrophages (additional step for macrophages take to remove material and ready to kill again)
List contents of primary and secondary granules of neutrophils and describe their functions.
PRIMARY GRANULES:
Hydrolases: breaks covalent bonds by adding water, important for degrading dead bacteria or dead tissues.
Lysozyme: breaks down peptidoglycan in Gram-positive bacteria. Found in many secretions in the body.
Defensins: small cationic proteins that kill bacteria, especially Gram-positive bacteria. Also called antimicrobial peptides.
These are 29-42 amino acids long. Have hydrophobic outside and hydrophilic interior and insert into a membrane and form a pore.
Myeloperoxidase: an enzyme that has an important role in the oxygen mediated killing mechanism
SECONDARY GRANULES
Lysozyme: like in primary granules
Lactoferrin: chelates iron – bacteria need iron for survival
Collagenase: degrades connective tissue, so it can move through to the site of inflammation
Compare fates of neutrophils and macrophages after phagocytosis.
Neutrophils die and lyse after extended phagocytosis, killing, and digestion of bacterial cells. This makes up the characteristic properties of pus.
Macrophages egest digested debris and allow insertion of microbial antigenic components into the plasma membrane for presentation to lymphocytes in the immunological response.
Compare and contrast M1 and M2 macrophages
Classical activation-M1 cells: KILLS
Increased: size, movement, membrane activity, lysosomal enzymes, phagocytosis, bactericides activity, MHC class II expression, No production
Alternative activation- M2 cells: REPAIRS
Increases tissue repair, increased MHC class II expression, reduced microbial killing
Explain how aged and/or dying neutrophils are removed from the body
As neutrophils age they express changes in their surface that send “eat me” messages to monocytes.
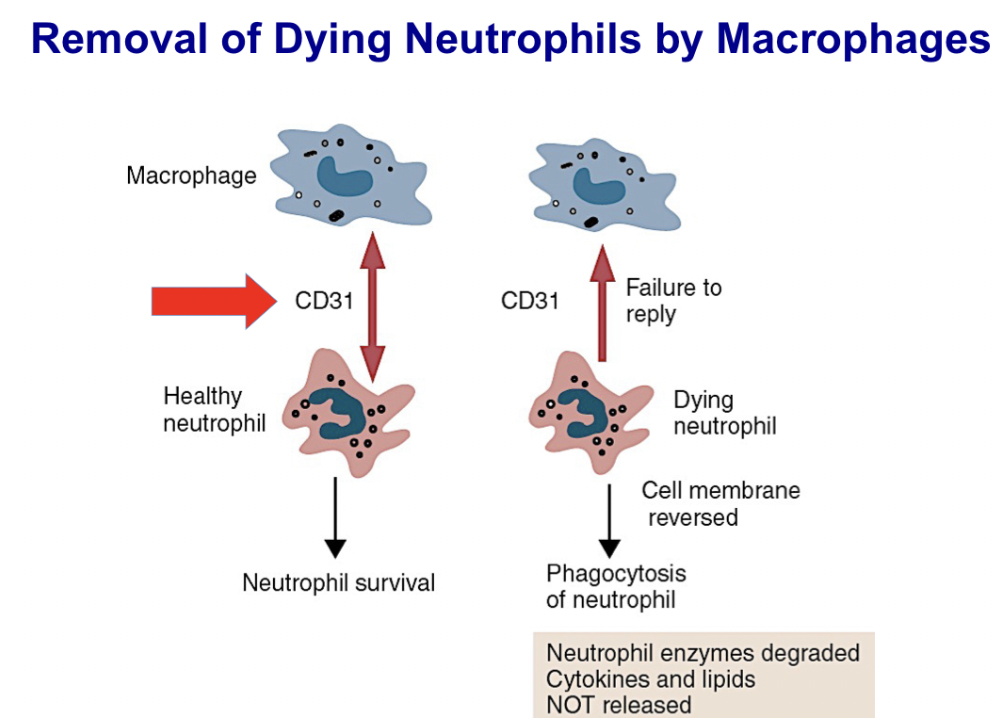
List three ways the complement cascade is initiated and molecules responsible for initiation in each
- The complement pathway may be activated through three different pathways: two innate pathways, the alternative pathway and the lectin pathway, and one adaptive pathway, the classical pathway.
-Lectin pathway uses MBL to get to MASP-2; Alternative uses C3bBb; and Classical uses C4b2b to get to C3, needs antibody to bind to C1q
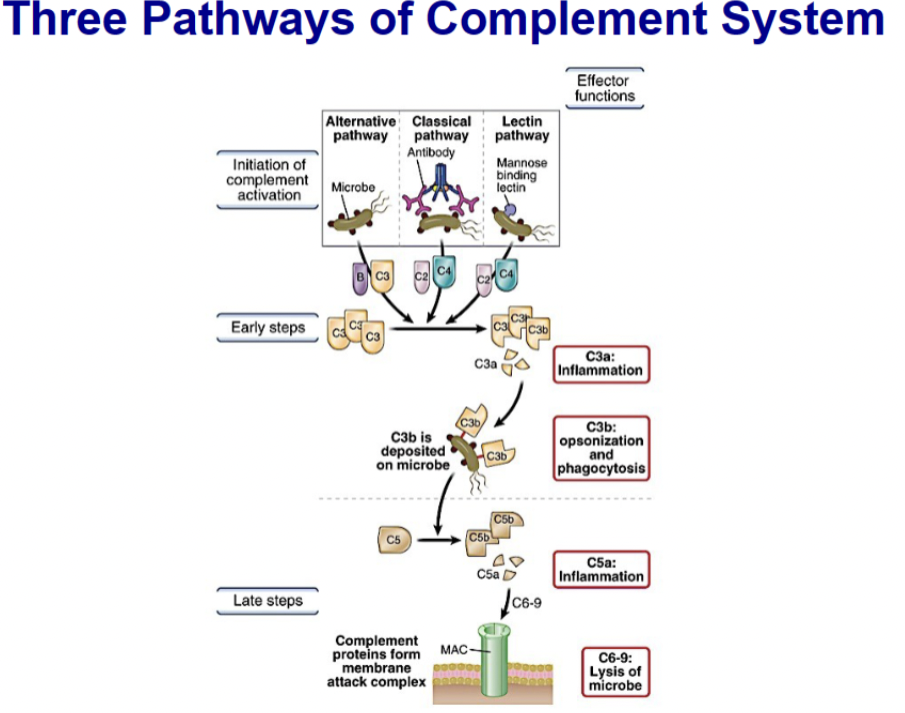
Compare and contrast the three pathways of complement activation, including initiation, type of immunity involved, where they diverge and where they converge
Differ in the Initiation of the cascade
Pathways same from C3 through the terminal pathway and formation of the membrane attack complex (MAC)
Alternative and MBL pathways are part of the innate defense
Classical pathway requires adaptive immune response to initiate (requires IgM, IgG)
Terminal pathway is same in all three pathways; Cleavage of C5 initiates the terminal pathway
From here on enzymes are not involved
Leads to formation of membrane attack complex (MAC)
List the classes of antibodies that can fix complement and explain why IgM is the most efficient in activating classical pathway
IgM is more efficient at activating the classical pathway of complement than IgG: One pentameric IgM bound to Ag can initiate complement cascade, Two IgG molecules in close proximity are required to activate C1
List three ways complement contributes to eliminating microbes
The complement system is a network of interacting pattern-recognition proteins, proteases, serum proteins, receptors, and regulators that kills invaders fast. The major complement proteins bind covalently (and hence irreversibly) to the surface of invading microbes and then destroy them. The complement system is activated by the presence of either pathogen-associated molecular patterns (PAMPs) or by antigen-bound antibodies. Because the complement system is so potent, it must be tightly regulated and controlled.
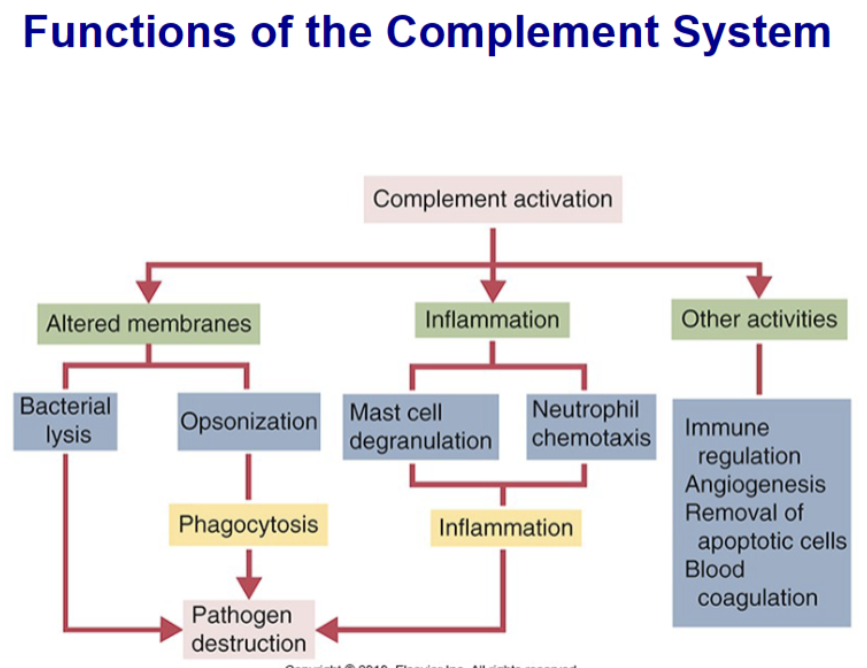
List complement components that are important in opsonization, chemotaxis, and vascular changes in inflammation
Opsonization= Molecules that coat bacteria in this way and promote phagocytosis are called opsonins. This word is derived from the Greek word for “sauce,” implying that they make the bacterium more attractive to neutrophils; C3b
Chemotaxis= Neutrophils crawl directly toward invading organisms and damaged tissues attracted by chemotactic molecules. These chemoattractants diffuse from sites of microbial invasion and form a gradient. Neutrophils crawl toward the area of highest concentration—the source of the material
Vascular changes= Neutrophil granule proteins enhance monocyte adherence to vascular endothelium, trigger macrophages to secrete cytokines, and activate dendritic cells, thus promoting antigen presentation.
Explain, including the molecules involved, why the alternative complement pathway is not initiated on mammalian cells, but it is on bacterial cells
- “CD55, or decay accelerating factor, is a glycoprotein expressed on red blood cells, neutrophils, lymphocytes, monocytes, platelets, and endothelial cells. CD55 binds to the convertases and accelerates their decay. Its function is to protect normal cells from complement attack.”
- Complement receptors are found on mammalian cells (RBC, lymphocytes, neutrophils) to protect own cells and to “mark” pathogens for destruction
- our cells are coated with factor H to inactive C3 when not needed, checks and balance system; I activated C2, also on our cells, again as a checks and balance system
Describe properties of cytokines
Cytokines secretion is a brief, self-limited event
Produced only when needed
Many individual cytokines are produced by multiple diverse cell types
Individual cytokines may act on many different cell types
Have multiple effects on the same target cell
May act locally and/or systemically
Have redundant functions
Actions can be synergistic
Act by binding to specific membrane receptors on target cells
Expression of cytokine receptors is regulated by multiple signals
Often influence the synthesis and action of other cytokines
Cellular responses to cytokines require new mRNA and protein synthesis
Tightly regulated and feedback inhibitory mechanisms exist
Describe pathways that trigger cytokine release
Three of the most important pathways that trigger cytokine release:
the binding of antigens to their receptors on T and B cells
the binding of PAMPs to pattern-recognition receptors on sentinel cells
the binding of antibodies to Fc receptors on phagocytic cells.
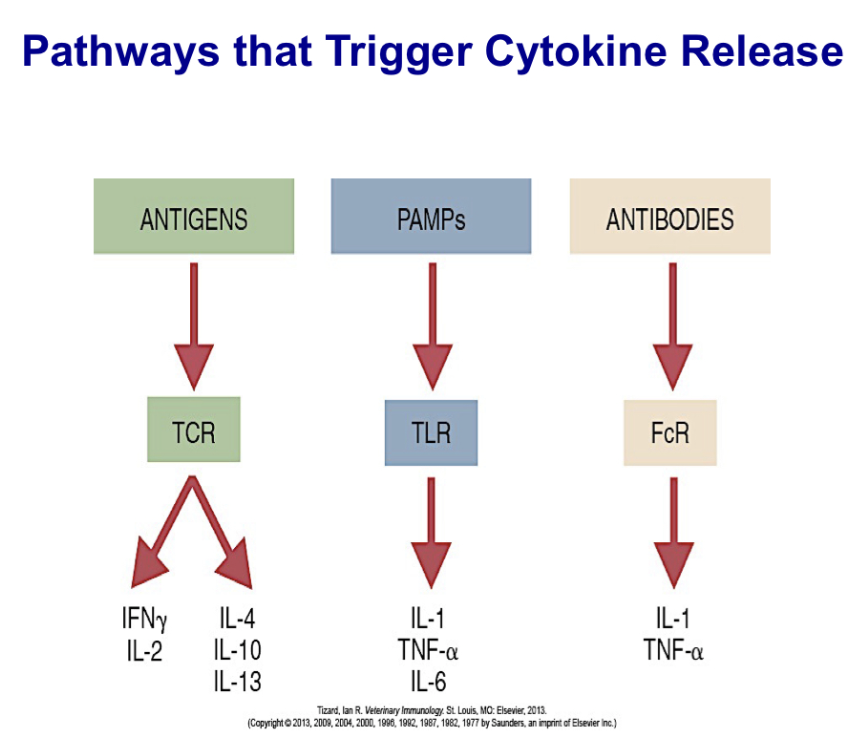
Broadly explain signal transduction events that lead to production of cytokines
Signal transduction mediated through T cell antigen receptors generates three transcription factors: NF-AT, NF-κB, and AP-1. When TCRs cluster, they activate several protein kinases. The most important of these is called ZAP-70. This in turn triggers three signaling pathways, and with appropriate costimulation, generates multiple transduction factors. The jun-fos heterodimer (AP-1) is required to stimulate the genes for cytokines and their receptors. The final results of the stimulus include cell division or apoptosis as well as cytokine production.
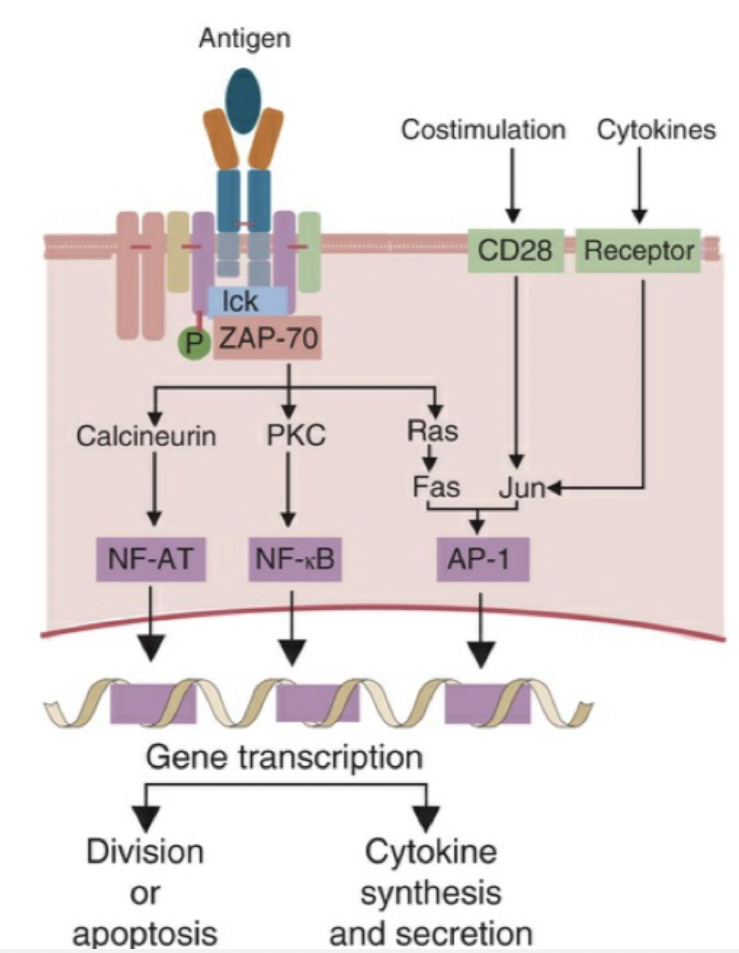
List major cytokines that mediate innate immunity and give a brief description of each
IL-1, IL-6 and TNFα:
Proinflammatory cytokines
IL-12:
Produced by antigen presenting cells in response to bacteria and viruses
Activate NK cells to be more efficient killers
Induce IFNγ production
Stimulate differentiation of TH1 cells
Type I interferons:
Antiviral activity
Examples: Interferon alpha (IFNα) , and Interferon beta (IFNβ)
List major cytokines that mediate adaptive immunity and give a brief description of each
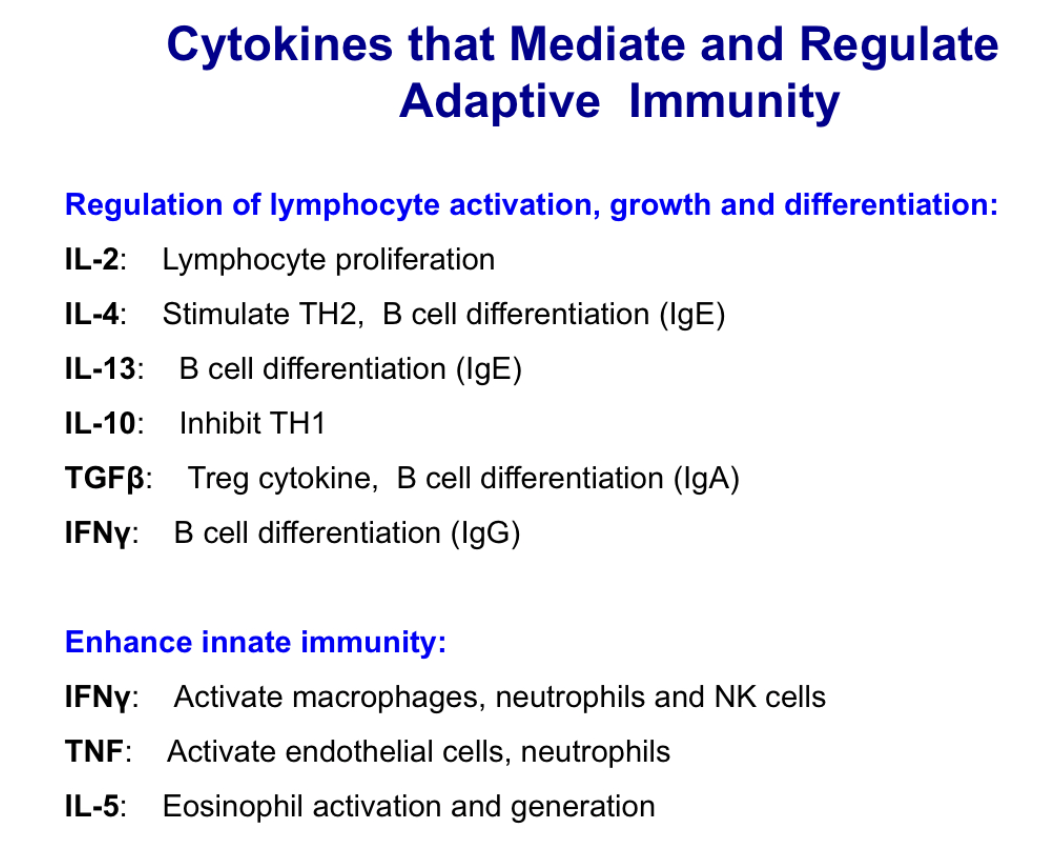
List major cytokines that mediate hematopoiesis.
Regulate growth and differentiation of bone marrow progenitor cells
Produced during both innate and adaptive immune responses
Clinically important hematopoietic cytokines:
Erythropoietin: Stimulates production and differentiation of RBC
Thrombopoietin and IL-11: Stimulate platelet production
IL-3: Stimulates eosinophil differentiation during parasite infection/allergic response; Antagonists used in eosinophilia and asthma
GM-CSF: Stimulate differentiation of neutrophil and monocyte
G-CSF: Stimulate differentiation of neutrophils
M-CSF: Stimulate differentiation of monocytes
Define an antigen.
An antigen is foreign molecules that trigger an immune response
Describe properties of a good antigen
Large, complex, and foreign , stability.
ability of an antigen to elicit immune response also depends on: Route of administration, amount of antigen administered, genetic makeup of the immunized animal
Ex: capsid proteins, amino acids
Define hapten and carrier
Haptens are small molecules which cannot be immunogenic on their own, so they are used as antigens in the way that they are made antigenic by linking them to large proteins.
The antigenic molecule to which the haptens are attached is called the carrier.
Briefly explain an immunological response to a hapten- carrier and give an example of a clinically relevant situation of hapten-carrier complex
Small molecules of <1000 daltons are far too small to be appropriately processed and presented to the immune system.
If these small molecules are chemically linked to a large protein molecule, new epitopes will be formed on the surface of the larger molecule and this complex when injected in an animal will trigger immune response.
Example: Penicillin allergy
poison ivy allergic contact dermatitis:
The toxic component of poison ivy plant, called urushiol, can bind to any protein which it comes into contact.
It binds with skin proteins of a person who rubs against the plant
The modified skin proteins are treated as foreign and attacked by lymphocytes.
It results in a skin rash called allergic contact dermatitis
Define autoantigens and give a few examples
Sometimes an animal may mount immune responses against normal body components, referred to as autoimmune responses.
Antigens that induce such responses are called autoantigens.
examples include: Thyroglobulin, myelin, mitochondrial proteins, etc.
List three professional antigen presenting cells and give brief description of how/when they present antigen
Dendritic cells (DCs) - activates naive T cells and trigger primary immune response; present primarily in epithelial tissues and lymphoid organs
Macrophages - present antigen to memory T helper cells
B-cells - secondary immune response; present antigen to memory T helper cells
Explain the differences between immature and mature dendritic cells
Immature dendritic cells: Found in tissues
Antigen uptake/processing - need enzymes to chop it up and load on MHC inside cell
low surface MHC II
High intracellular MHC II
High FcR
Low CD40, 80, 86
Low IL-12
Mature dendritic cells: Found in Lymphoid tissues
Antigen presentation - presented on surface, ready to present to T cells
high surface MHC
Low FcR
High CD40, 8-,86
High IL-12
DC-SIGN
Define Langerhans cells
Specialized dendritic cells found in skin, looking for any antigen
Differentiate between follicular dendritic cells and dendritic cells (their origin, antigen presentation, and locations)
They do not migrate
located in lymphoid follicles (B-cell area)
lack MHC II molecules on their surface
carry many complement and Fc receptors
List the steps describing antigen presentation through the endogenous and exogenous antigen presentation pathways
Endogenous Pathway of MHC I (endogenous, taking viruses that are infecting cell and presents it as an antigen)
Ubiquitin binds to the cytoplasmic protein and targets it to proteasome.
Proteasome is a cellular organelle that is a hollow cylinder and degrades proteins into short peptides (about 8-15 aa).
Transporter for Antigen Processing (TAP) bind cytosolic peptides from the proteasome and transport them to the lumen of the endoplasmic reticulum (ER).
Peptide is trimmed to 9 aa by the ERAP (ER resident aminopeptidase) and then binds to an empty MHC I in the ER.
MHC I plus peptide move to Golgi and then transported in exocytic vesicle to cell surface. Antigen-MHC I available for recognition by CD8+ T cell.
Exogenous Pathway of MHC II
APC internalizes the antigen via phagocytosis or endocytosis
Lysosomal enzymes digest the antigen into short peptides
MHC molecule is synthesized in the rough endoplasmic reticulum
Alpha & Beta chains of MHC II come together and form a groove
Invariant chain fill the peptide groove while the MHC II is transported through the golgi.
Exocytic vesicle of the RER fuses with the endosome/phagolysosome containing the antigen peptides
The invariant chain is partially digested leaving a small peptide CLIP (class II associate li Peptide)
CLIP is removed by DM, a peptide exchanger molecule, and antigen peptide binds to the MHC II groove.
MHC II with antigen in groove is transported to membrane and presented
Compare and contrast the type of antigens that are predominantly presented via the endogenous and exogenous pathways
MHC I molecules present endogenous peptides which are derived from proteins manufactured by or within the cells. (Endogenous)
MHCII present ONLY on antigen presenting cells (exogenous)
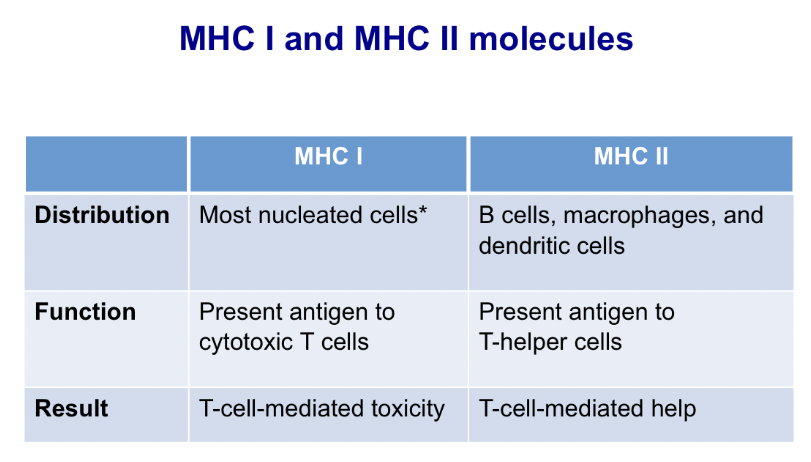
Explain MHC restriction and heterozygote advantage
Only antigen fragments that can bind in the groove of a MHC molecule can trigger an immune response. This is called MHC restriction.
MHC heterozygotes are at an advantage because they can respond to a greater range of antigens.
List examples of MHC-associated resistance or susceptibility to various diseases
Association between BoLA-Aw7 and resistance to bovine leukosis (bovine leukemia virus).
BoLA-A*16 and resistance to mastitis.
BoLA DR locus and resistance to Dermatophilus sp.
ELA-A9 and susceptibility to equine recurrent uveitis.
ELA-A3, ELA-A15, ELA-Dw13 and development of sarcoid tumors (likely induced by bovine papilloma virus)
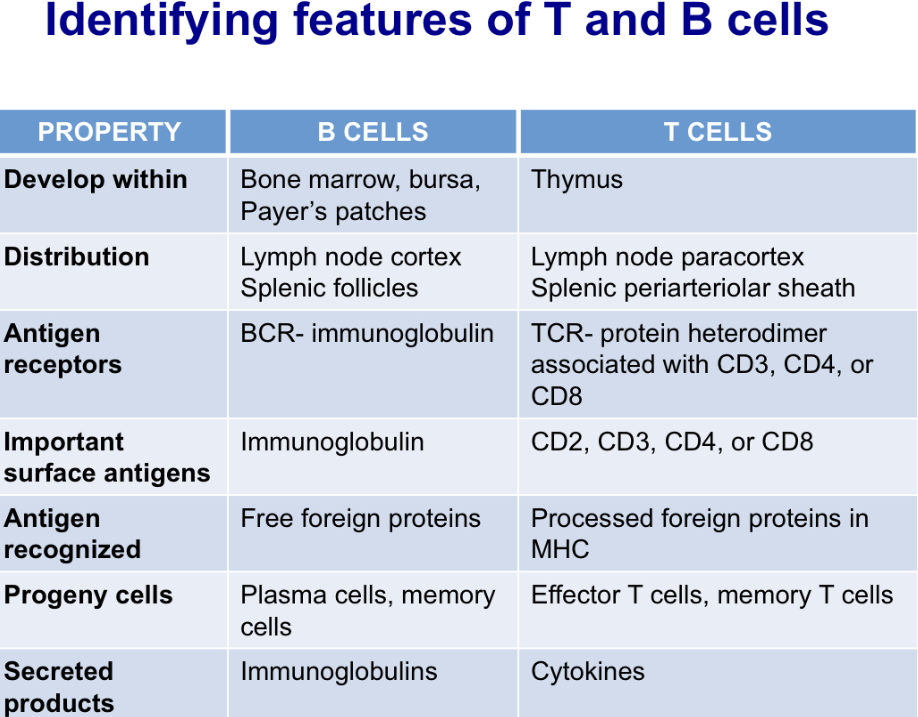
Describe identifying features of T and B cells
List steps in the maturation of lymphocytes and explain positive and negative selection
Negative selection: The killing of T cells that have the potential to react to self antigens. A key mechanism in the prevention of autoimmunity.
Positive selection: enhanced proliferation of cells within the thymus that can respond optimally to foreign antigen.
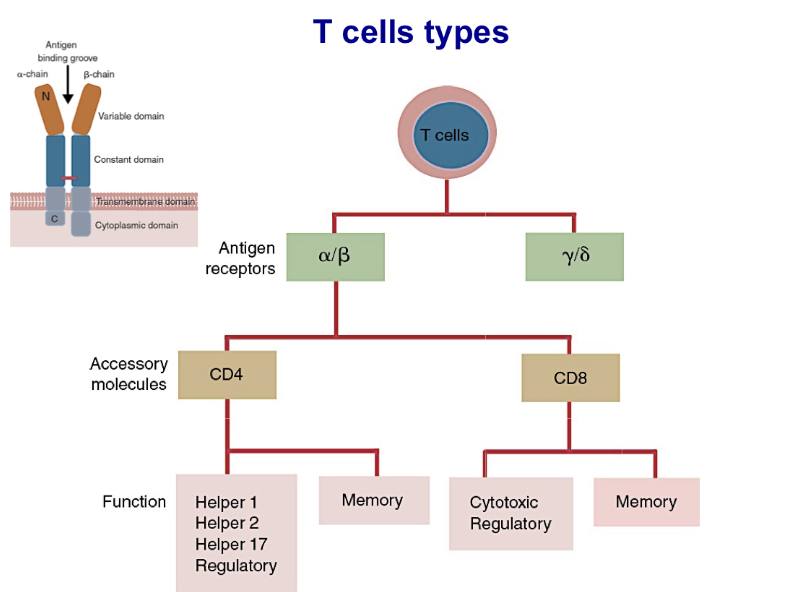
List different types of T cells based on their antigen receptors, accessory molecules and function
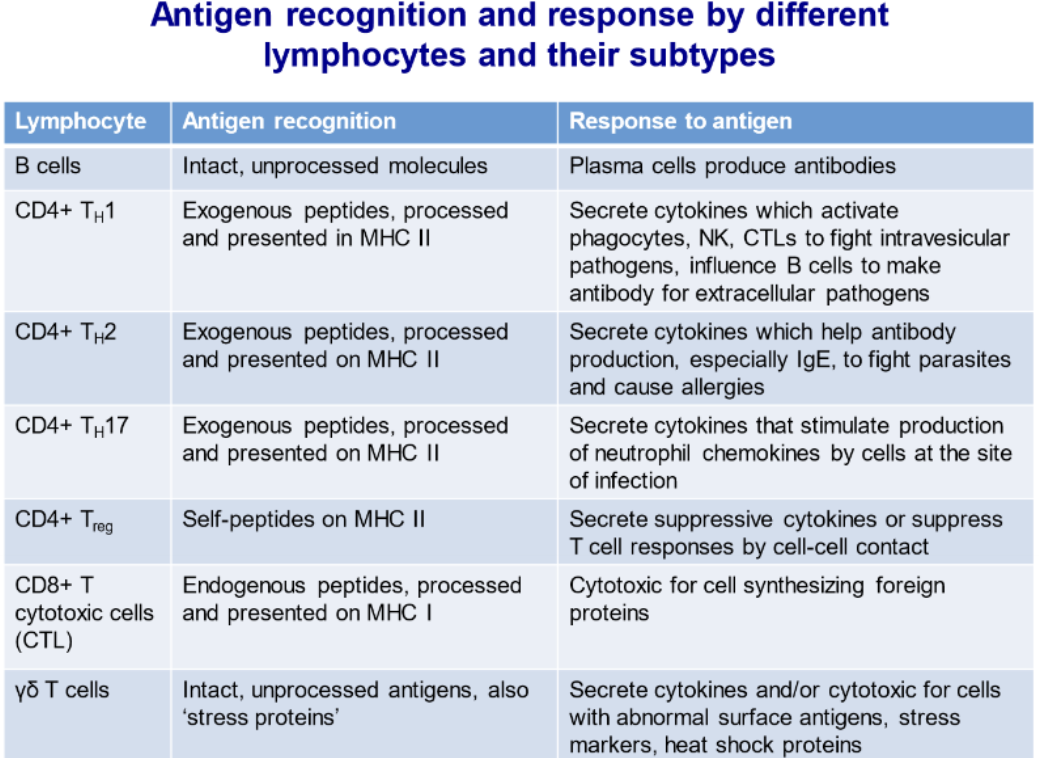
Describe differences in antigen recognition and responses by B cells and different subtypes of T cells
Name three major receptors of NK cells and explain their roles in the activity of NK cells
the three major receptor types found on mouse NK cells.
Ly49 recognizes MHC class I molecules and suppresses NK cytotoxicity.
NKG2D is a receptor for molecules such as MIC-A and MIC-B. These molecules are commonly expressed on stressed, virus-infected, or tumor cells.
CD16 binds immunoglobulins and triggers target cell death by antibody-dependent cellular cytotoxicity. Remember that in other mammals, Ly49 may be replaced by KIR receptors
Describe the structure of TCR Complex
TCR complex is made up of the TCR alpha and Beta chains, which are responsible for antigen recognition and the CD3 complex and Z homodimers, which are required for signal transduction
Explain differences in TCR complex of a T helper cell and a cytotoxic T cell
If T helper cell (antigen presenting) it will have a CD4 and will also have the CD3 complex; MHC 2. Thus making it a T helper cell. If it is a cytotoxic cell (abnormal cell) it will have a CD8 and CD3 complex; MHC 1
Explain how T cell - APC interaction results in activation or inactivation of T cell response
Antigen presenting cell is loaded with antigen, and has MHC class II and interacts with T cell receptor. Once interaction happens, there is upregulation of Cd 154 (receptor) on surface of T cell. It binds with Cd 40 on antigen presenting cell and when that happens 2 things happen:
upregulation of Cd 28 on T cells that interacts with Cd 80/86. All costimulatory molecules on antigen presenting cells. Now T cell gets activated.
Starts making IL-2. IL-2 are cytokines that activates T cells (triggers T cells and causes differentiation of T cells to make more of its kind).
After 72 Hours another Cd molecule from T cell is produced, Cd152 and is taken to the surface. CD 80/86 binds to cd152 instead of cd28 and leads to inactivation. When this happens formation of IDO (indolamine deoxygenase) leads to destruction of tryptophan (essential amino acid) so T cell response is terminated
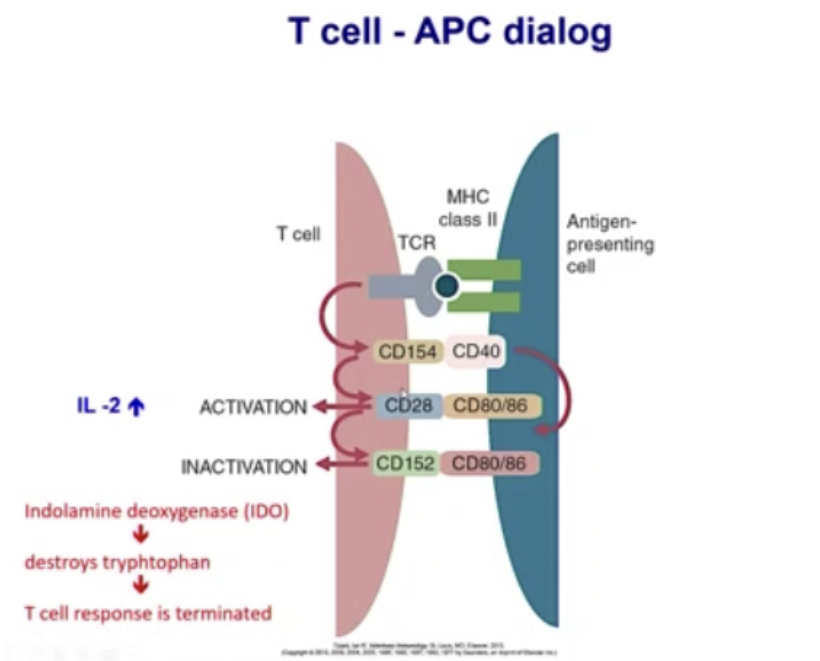
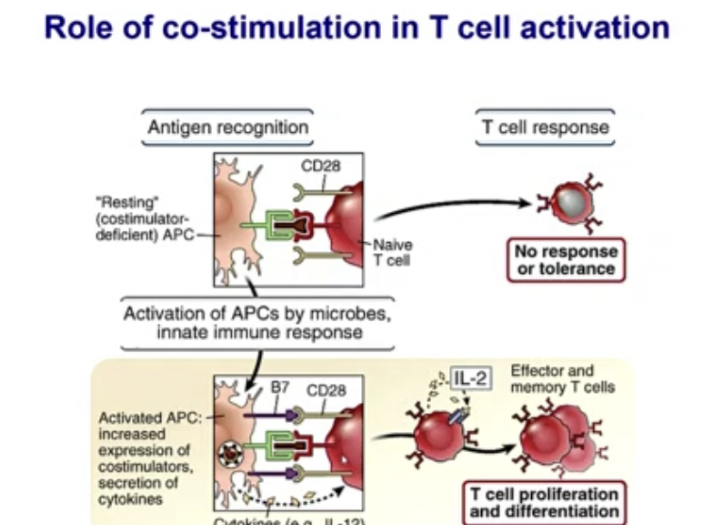
Explain importance of costimulation in T cell activation
Costimulatory molecules (B7 is one, another name for CD 80/86) helps T cells get stimulated.
If there is no costimulation, T cells do not respond well
Name different cytokines and transcriptional factors involved in development of various T cell subsets
TH1: (IL-12+IFNY) + T-Bet= [IFNY, THFA, IL-2]: Function= T-cell responses.
TH2:(IL-4, IL-33, TSLP) + GATA3 = [IL-4,5,9,13]: Function: B-Cell responses
TH17:(IL-6, IL-23, TGFB) + ROR-yt = [IL-17,21,22]: Function: Increased Inflammation
Treg:(IL-10,TGFB) + FoxP3 = [IL-10,35 and TGFB]: Function: Decreased Inflammation
List roles of different T cell populations in immune responses to various microbes
Note: all T-cells must be bound to one of the cytokines in order to activate and mature into T-Cells.
TH1+ Receptor site= Active Cell+IFNY—>targets Macrophage
TH2+Receptor site= Active Cell+[IL4,5,13]----> Targets Esonophils
TH17+ Receptor site= Active cell+[IL17,22]----->Targets Neutrophils
![<ul><li><p><strong><u><span>Note: all T-cells must be bound to one of the cytokines in order to activate and mature into T-Cells.</span></u></strong></p></li></ul><p></p><ul><li><p><strong><span>TH1+ Receptor site= Active Cell+IFNY—>targets Macrophage</span></strong></p></li><li><p><span>TH2+Receptor site= Active Cell+[IL4,5,13]----> Targets Esonophils</span></p></li><li><p><span>TH17+ Receptor site= Active cell+[IL17,22]----->Targets Neutrophils</span></p></li></ul>](https://knowt-user-attachments.s3.amazonaws.com/ebbfd5c3-b26b-420b-9251-484fd7e1408a.jpeg)
Describe antigen recognition and response by B cells and contrast it with that by T cells
BCRs recognize free antigens while TCRs recognize MHC bound antigen
TCRs require CD3 to form a antigen receptor complex; B cells use CD79
Discuss the role of TH cells in activation and differentiation of B cells
All antigen receptors on a single B cell are identical, each B cell can bind only one antigen. As a result, B cells can active Th cells with 1/1000 of the antigen required to activate macrophages
When helper T cells “help” B cells they promote several different B cell activities. These signals result in increased expression of IgM BCR and MHC class II, as well as receptors for IL-4, IL-5, IL-6, tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β), and start the process that leads to B cell division and differentiation into antibody-secreting cells
Explain why dendritic cells are important in the primary B cell response
Only dendritic cells can present antigen to naive T cells, so they are critical in primary immune response. B cells can present antigen but only in secondary immune response.
During a primary immune response, antigen is processed by a dendritic cell and presented to the helper T cell. During a secondary immune response, the B cell itself can act as an antigen-presenting cell. Co- stimulators, such as CD154 and CD28, engage serially to trigger IL- 4 secretion by the T cell and IL-4R production by the B cell
Describe antibody class switching and affinity maturation.
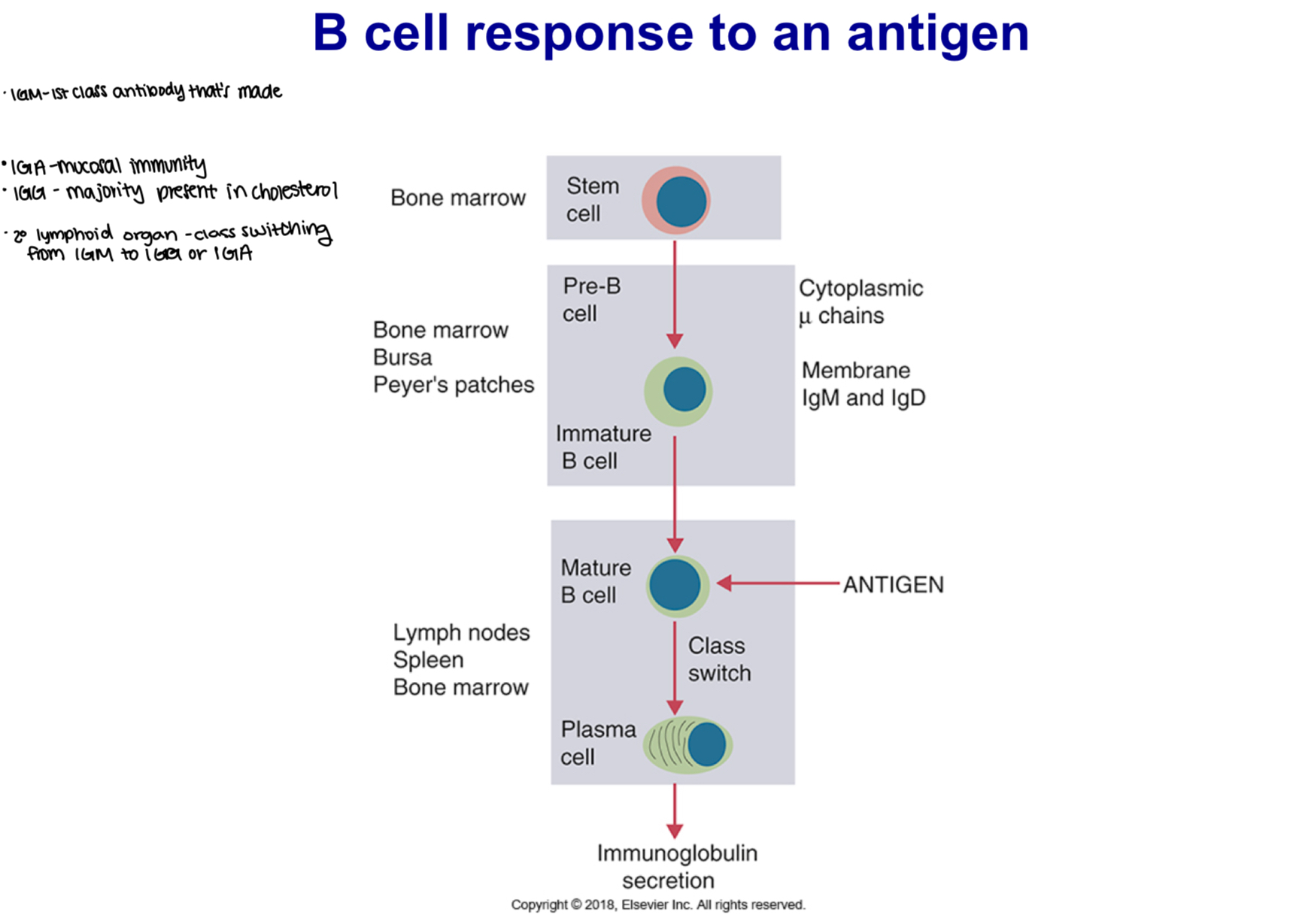
-IgM - 1st class antibody that’s made
-IgA - mucosal immunity
-IgG - majority present in cholesterol
-secondary lymphoid organ - class switching from IgM to IgG or IgA
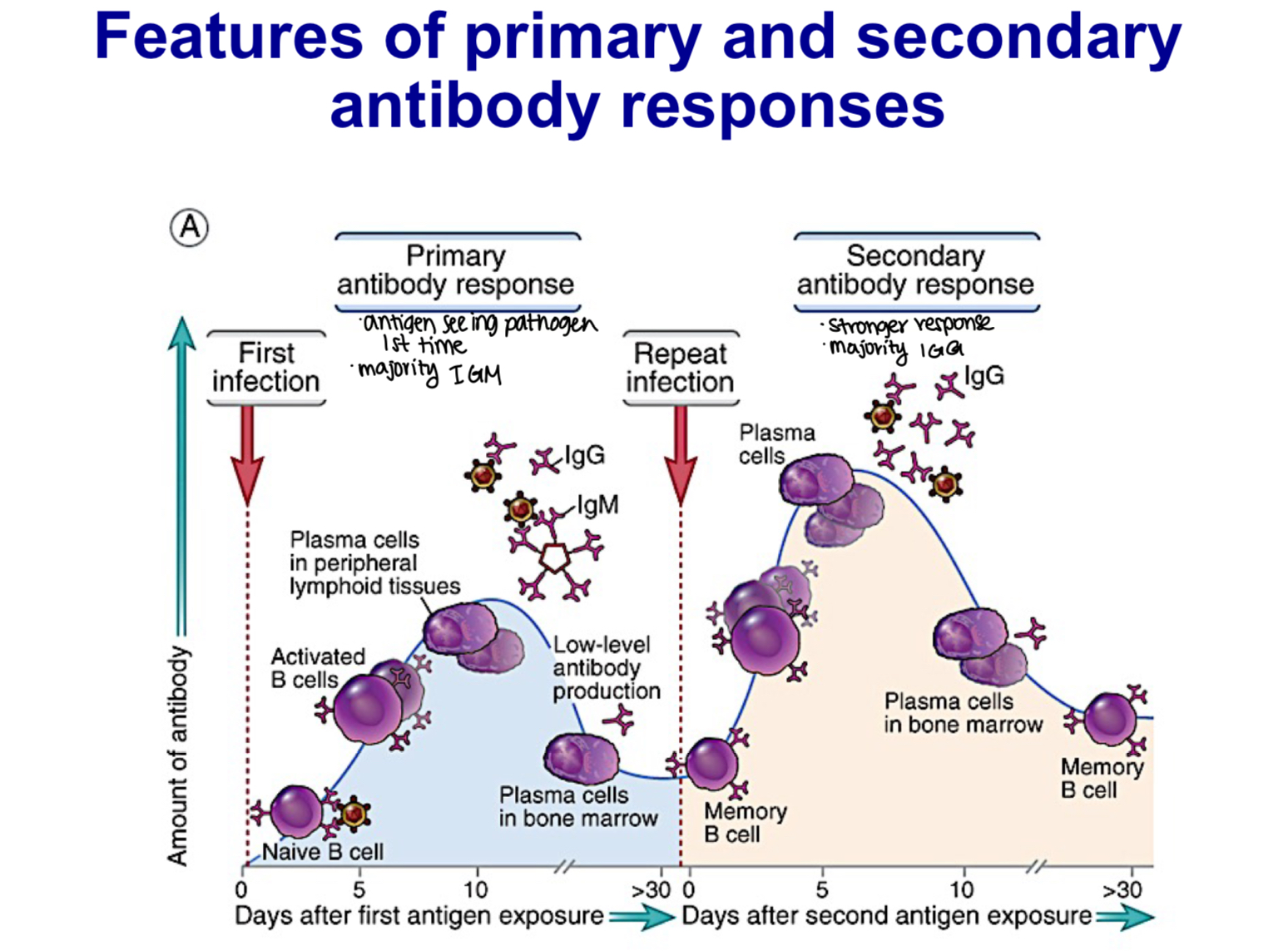
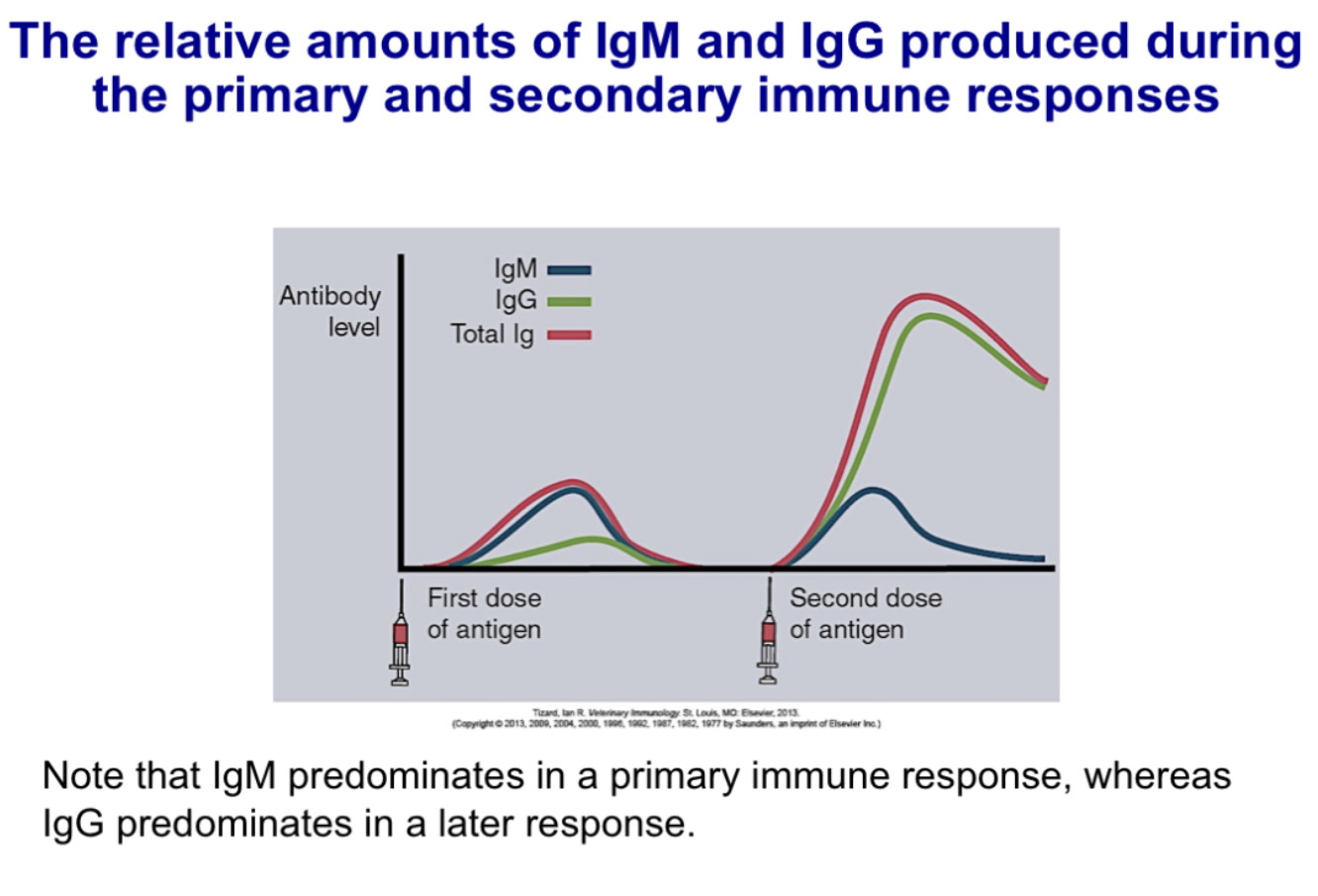
Compare and contrast primary and secondary immune responses in regard to the lag time, titer, antibody class and antibody affinity
-primary immune response:
5-10 day lag time
smaller peak response
usually IgM>IgG
lower avg. affinity, more variable
-secondary immune response:
1-3 day lag time
larger peak response
increase in IgG and, under certain situations, in IgA or IgE (heavy-chain isotype switching)
higher avg affinity (affinity maturation)
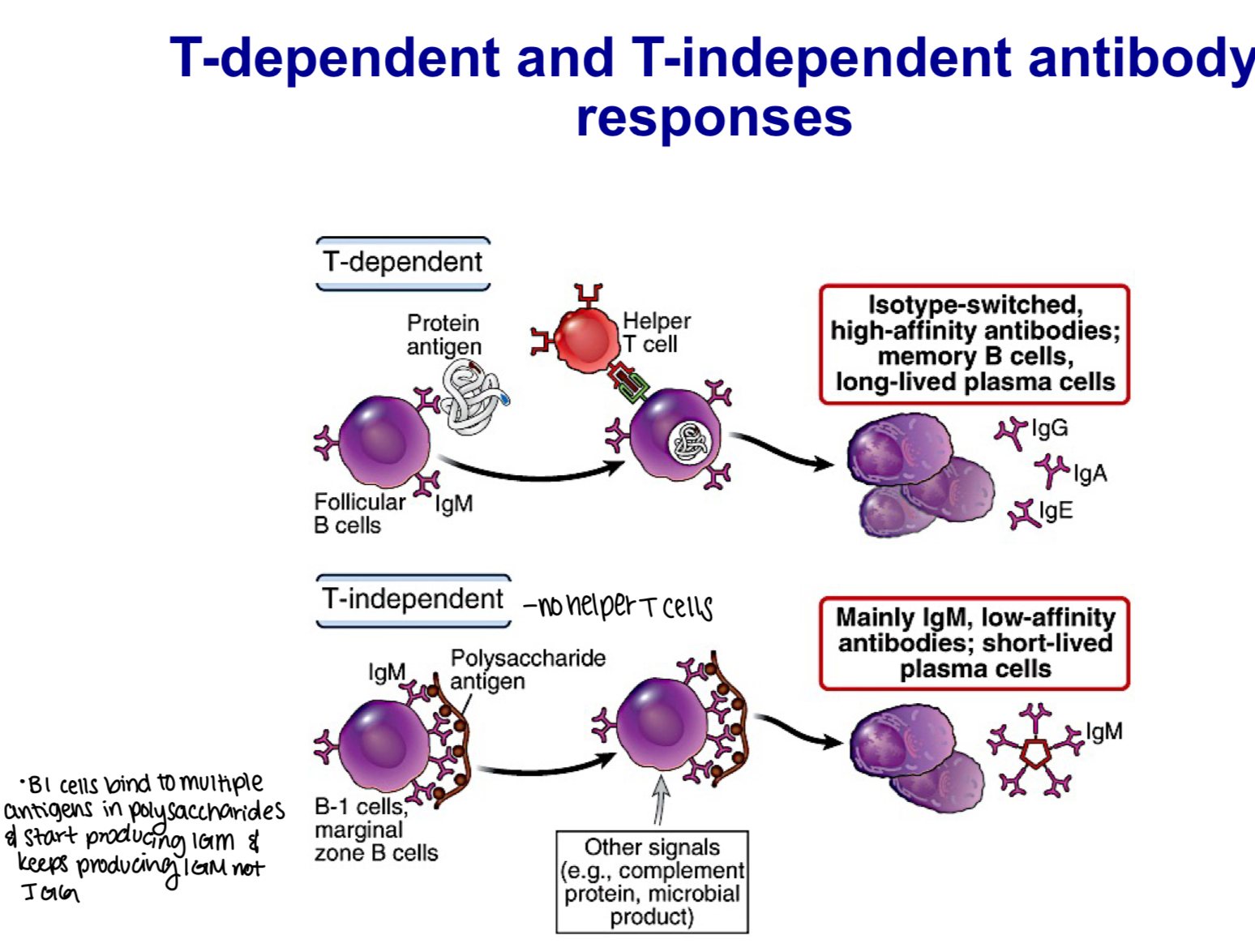
Differentiate between T-dependent and T-independent B cell responses
Draw the structure of an antibody molecule and label the following: heavy chains, light chains, constant domains, variable domains, Fab, Fc, antigen binding site, and hinge region.
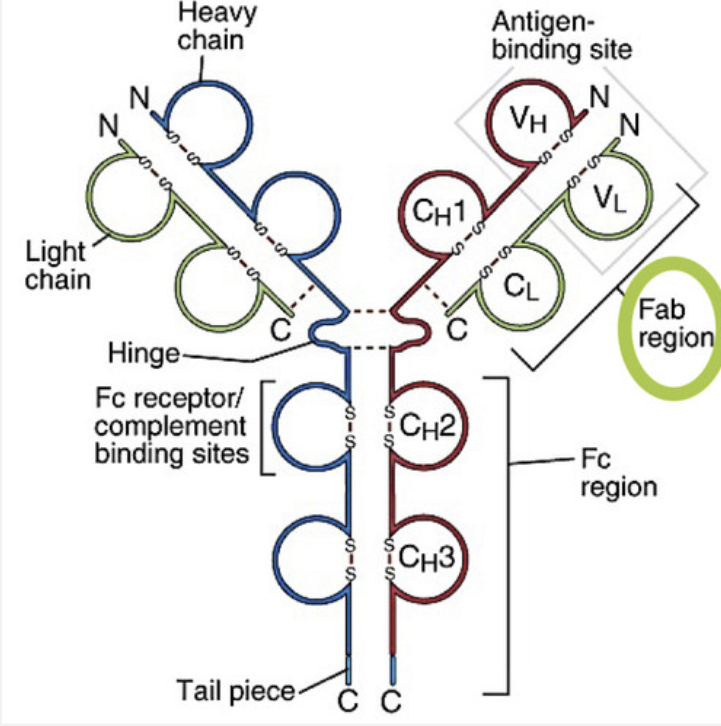
List different types of heavy and light chains of an antibody
-Two identical heavy chains
μ (mu), δ (delta), γ (gamma), ε (epsilon), α (alpha)
determine the class (isotype) of antibody
How many domains does heavy chain have?
4 domains: 1 variable domain, and 3 constant domains
What 4 domains make up heavy chain: VH, CH1, CH2, Ch3
Two identical light chains
each molecule has either λ (lambda) or κ (kappa)
all five classes of antibody use both kappa and lambda light chains
How many domains does a light chain have?
2 domains: 1 variable domain, 1 constant domain.
What 2 domains make up light chains: VL, CL
Define hypervariable regions (CDRs) of an antibody and its biological importance
Complementarity determining regions (CDRs) super variable region within the variable region. It is called this because this sequence of proteins determines the complementarity with the antigen bc it binds to the antigen
List five isotypes (classes) of antibody and discuss their characteristics and primary functions
1.Immunoglobulin G (IgG)
Four Heavy chain domains
found in blood stream and In interstitial fluid in tissues
Found in high concentration in colostrum of large domestic species
has a half life of 3 weeks and makes up 80% total serum
Functions include: complement activation, neutralization of viruses and toxins, opsonization, systemic immunity in newborn, antibody dependent cell mediated cytoxicity
2.IgM
pentamer
MW~900,000 Da
heavy chain has 5 domains
held together by disulfide bonds and J chain
can bind 10 identical epitopes
First class of antibody produced in a primary response
Functions include
Complement activation
Agglutination
3.IgD
IgD is found on B cell surfaces and has a regulatory function.
Found is found on the surface of immature lymphocytes.
Important in B cell development.
4.IgE
MW ~190,000 Da
Five domains in heavy chain
A lot of polysaccharide attached to heavy chain
Fc portion of IgE has high affinity for the Fc receptor on mast cell
Binding of antigen to the IgE on a mast cell can result in mast cell degranulation
IgE can also activate eosinophils
Most IgE is found on mast cells and the concentration in plasma is low
Functions:
Important in defense against helminthic parasites
Mediator of immediate hypersensitivity
5.IgA
Dimer, MW~360,000 Da
4 heavy chain domains
held together by a J chain
Most of it is found on mucosal surfaces
Functions:
Found on mucosal surfaces where it can neutralize toxins and block entry of pathogens
it is also found in mothers milk and helps provide intestinal immunity to neonate
Contrast relative amounts of antibody isotypes in primary and secondary immune responses
Describe the genetic basis of generation of diversity for antigen specificity
Diversity is needed on receptors of B cells and T cells. They are needed on the variable region where antigen is binding to receptors.
The confirmation of the receptor and antigen interaction due to charges, as well as other groups of amino acids, and the bonds: hydrophobic bonds, hydrogen bonds, electrostatic bonds, and van der waals bonds, are where the diversity is happening and it is what you need to know to understand this generation of diversity
Compare methods of generating immunoglobulin diversity and TCR diversity
TCR and immunoglobulin gene rearrangements are specific in that immunoglobulin genes are not rearranged in T cells, and TCR genes are not rearranged in B cells. Like immunoglobulins, the four peptide chains, α, β, γ, and δ, that make up the two types of TCR can bind many specific antigens.
They are able to do this because each consists of a variable region attached to a constant region. The diversity of TCR V regions is generated exclusively by gene recombination in contrast to the diverse mechanisms employed by B cells.
Methods of generation TCR diversity include:
VJ, VJJ, VDJ, and VDDJ gene recombination
base deletion
base insertion
combinatorial association
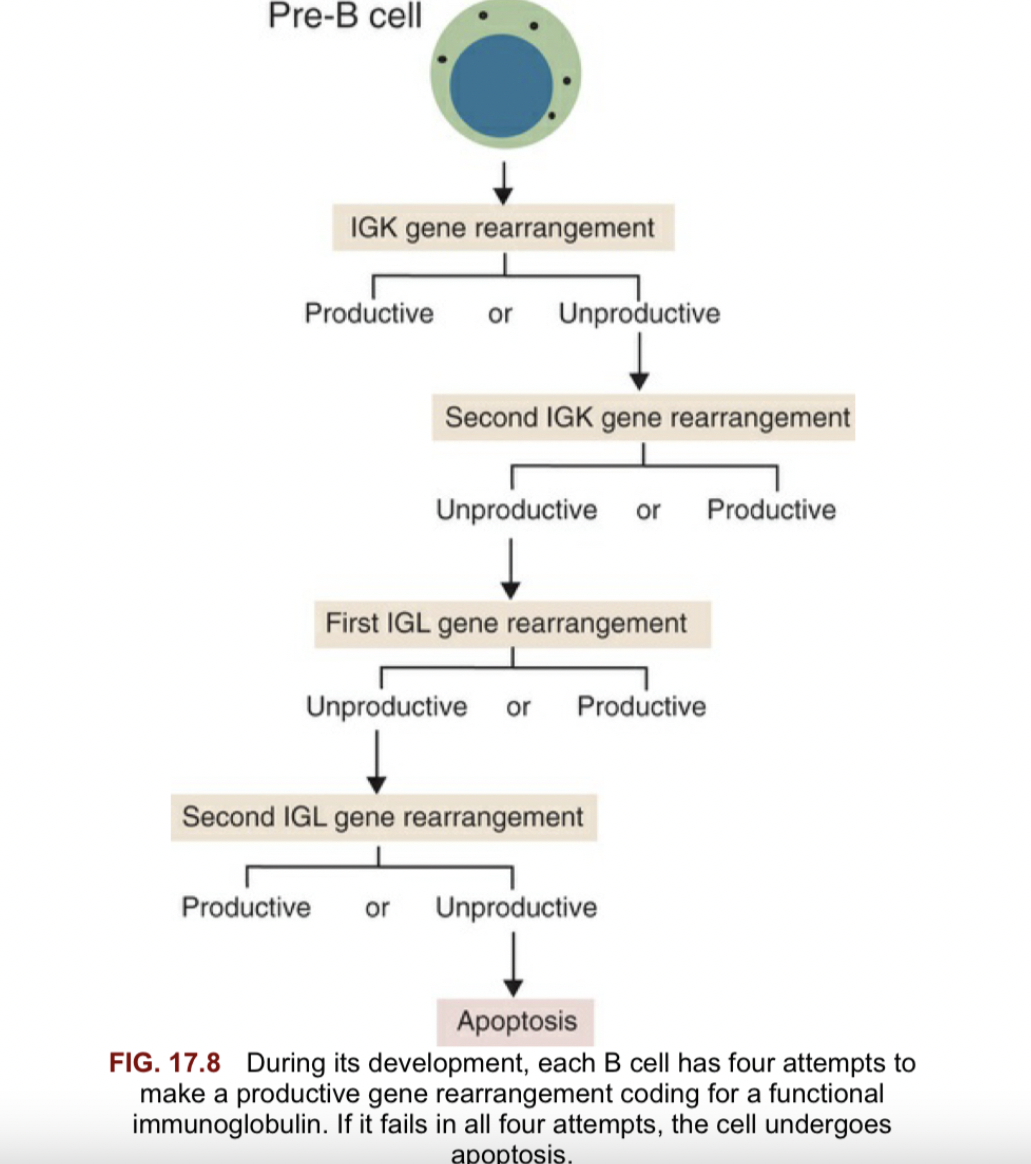
Explain the genetic basis for antibody class switching
During its development, each B cell has four attempts to make a productive gene rearrangement coding for a functional immunoglobulin. If it fails in all four attempts, the cell undergoes apoptosis
Define somatic mutation and explain how this leads to affinity maturation
A somatic mutation describes any alteration at the cellular level in somatic tissues occurring after fertilization. These mutations do not involve the germline and consequently do not pass on to offspring.
The name of the process is somatic mutation, whereas the result is affinity maturation
An average change of 1 AA in the V gene per cell division; those with increased affinity are retained whereas all others are destroyed. This is how Somatic mutation leads to affinity maturation.
CDR1 and CDR2 are formed by somatic mutation
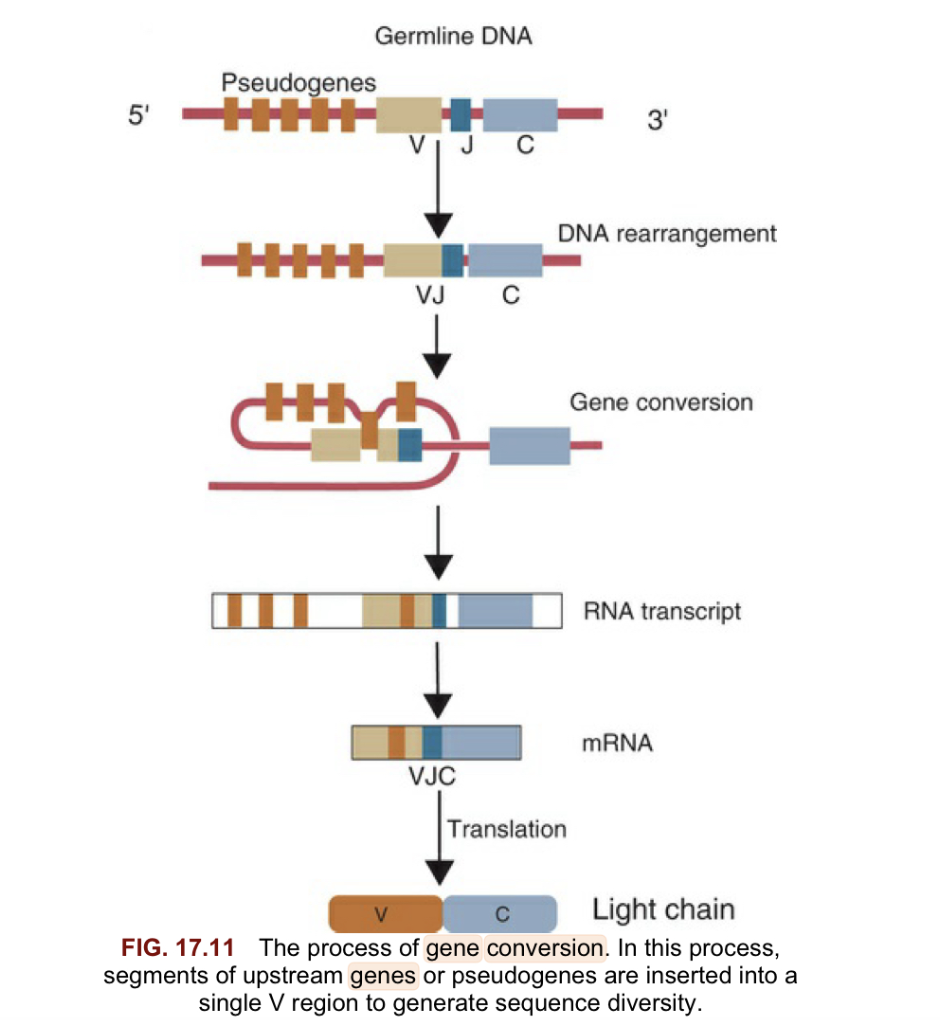
Describe gene conversion
Gene conversion is When segments of upstream pseudogenes are inserted into a single v region to generate sequence diversity important for species with only a few V genes reducing ability to achieve diversity with combination alone.
Define apoptosis and describe how it is induced and what happens in a cell that is induced to undergo apoptosis
-Apoptosis is programmed cell death or cell suicide that involves the controlled dismantling of intracellular components while avoiding inflammation and damage to surrounding cells
-Initiator caspases activateexecutioner caspases that subsequently coordinate their activities to demolish key structural proteins and activate other enzymes; major events are DNA fragmentation and membrane blebbing
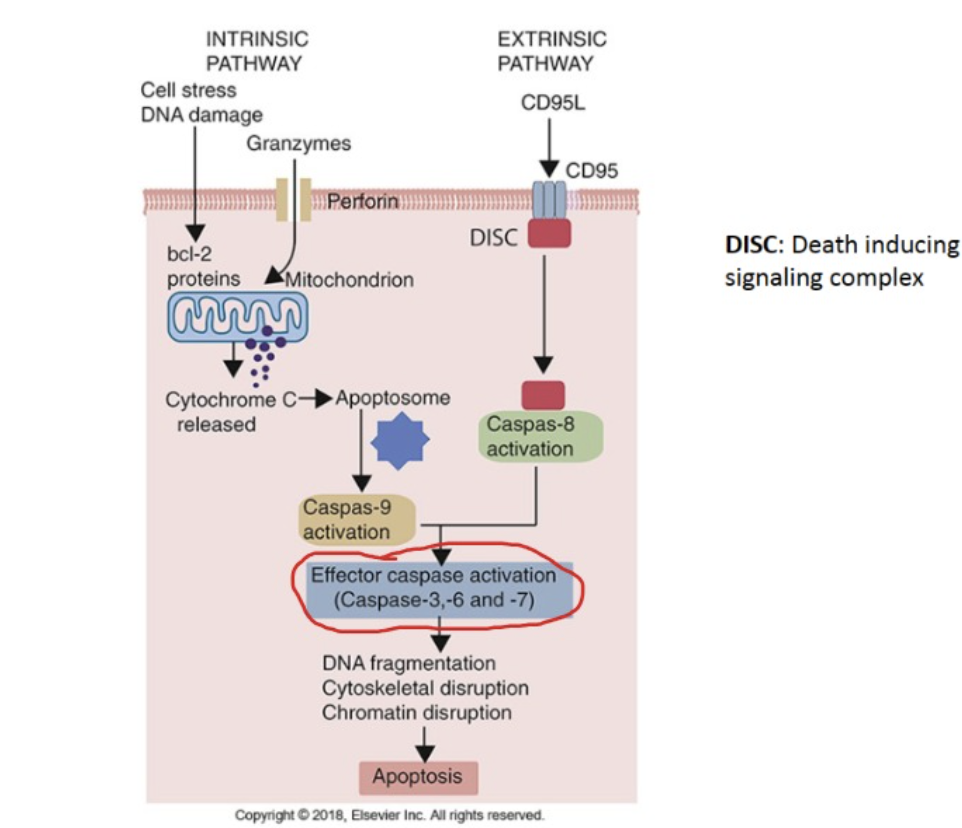
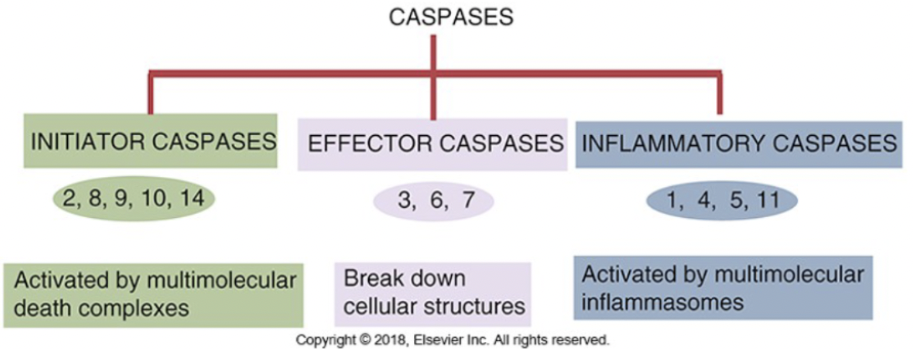
Discuss the role of caspases in apoptosis and inflammation
-Caspases are endopeptidases that are important in maintaining homeostasis through regulating apoptosis and inflammation
Explain the differences between apoptosis and necrosis
-Cell necrosis is characterized by membrane degradation, release of cytoplasmic contents, inflammation and involvement of larger area
-Apoptosis is programmed cell death that is controlled and dismantles the cell from the inside out without exciting the inflammatory response or damaging the cells around it
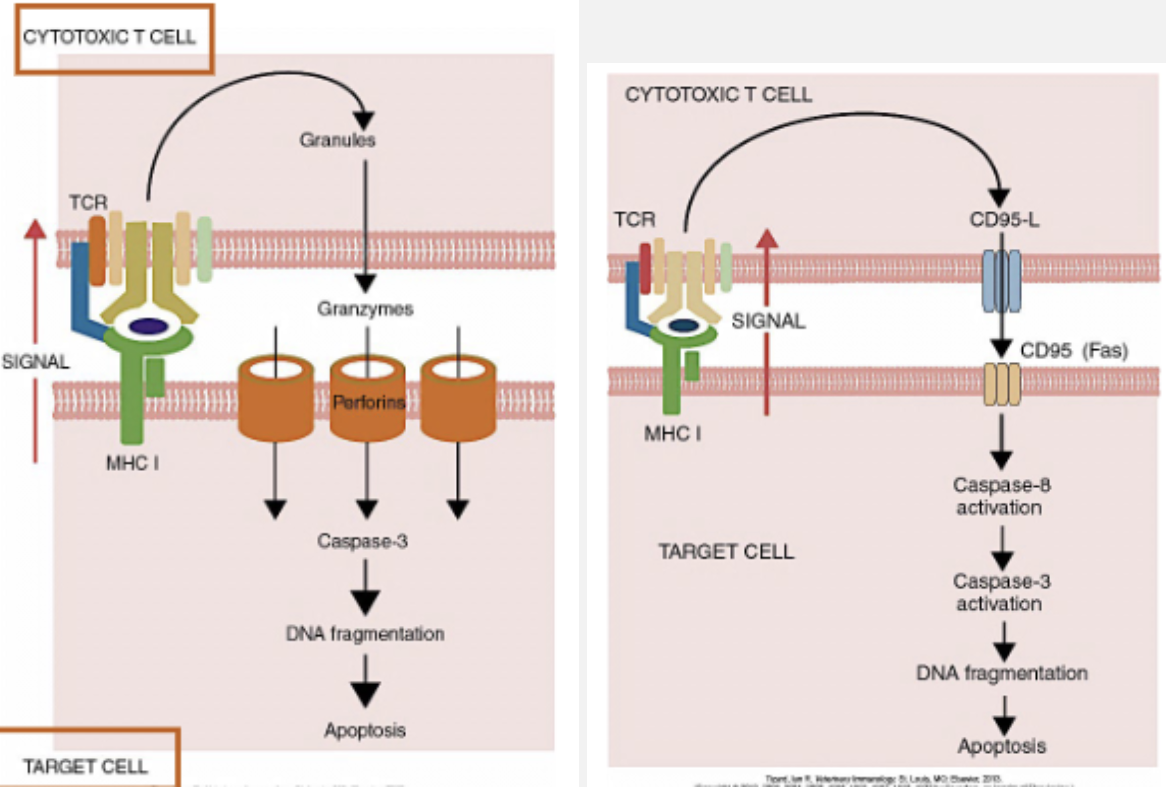
Describe mechanisms that CTLs use to kill target cells. For each, discuss the molecules involved and their functions
4 major types:
T cells: “T cell–mediated cytotoxicity is not the only way by which the immune system can destroy abnormal cells… These cytotoxic cells may include monocytes, eosinophils, neutrophils, B cells, and NK cells (Chapter 19).”
ADCC: “The mechanism of this antibody-dependent cell-mediated cytotoxicity (ADCC) is unclear. However, neutrophils and eosinophils probably release lethal oxidants and toxic granule contents. ADCC is slower and less efficient than direct T cell– mediated cytotoxicity, taking 6 to 18 hours to occur.”
Perforin and Fas pathways listed below:

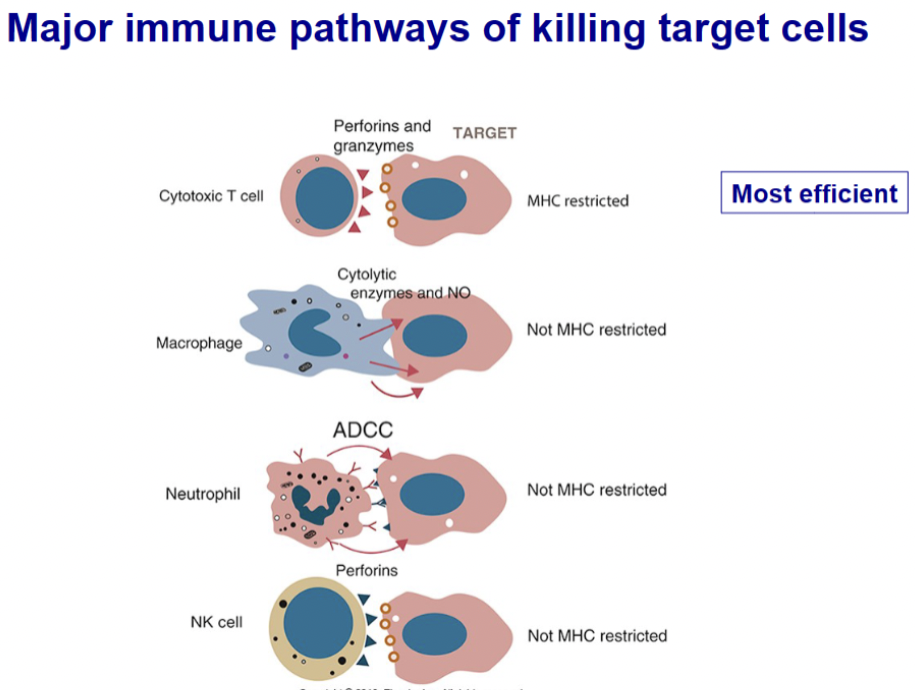
List major immune pathways of killing target cells
The four major pathways by which the cells of the immune system can kill nucleated target cells. These targets would normally be tumor cells or virus-infected cells. T cells and NK cells are directly cytotoxic. Macrophages secrete NOS and enzymes that kill nearby cells. Cells with Fc receptors act through ADCC mechanisms
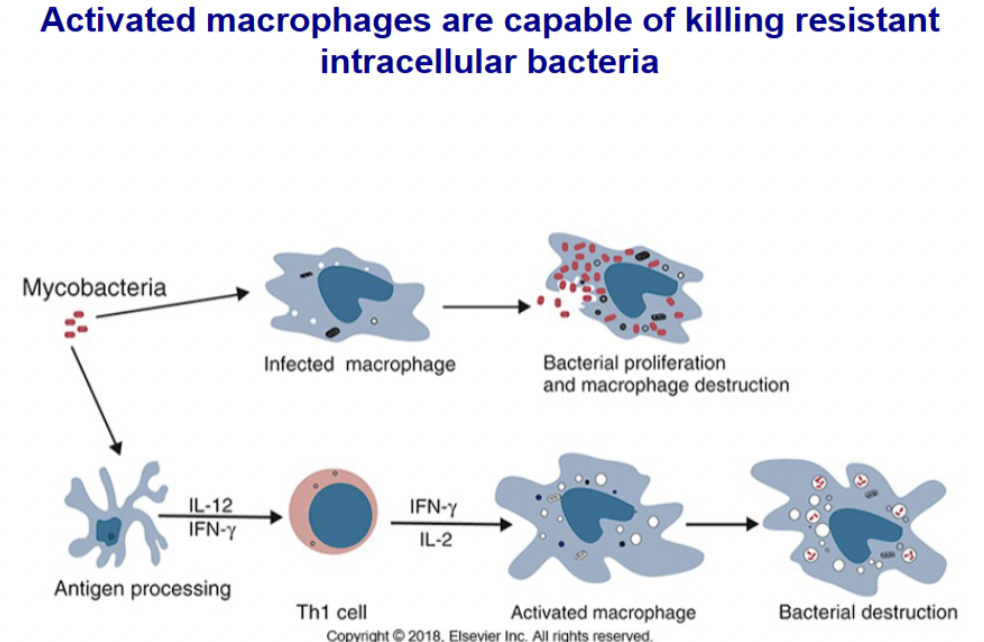
Discuss the role of activated macrophages in elimination of intracellular pathogens
Normal macrophages are killed by growing intracellular bacteria. IFN-γ and IL-2 released by Th1 cells can activate macrophages and enable them to kill otherwise resistant intracellular bacteria
Describe how parasites (protozoa, helminths and arthropods) get past Innate Immune system defenses: species resistance, physical/mechanical, chemical, and cellular
Species Resistance:
- Resistance to certain diseases to which other species are susceptible
-For many parasites – don’t cross species line; I.e. Dogs don’t get horse parasites.
•Examples: Lice – very host specific and site specific
-Utilize a specific species for sexual reproduction, but can use many for asexual reproduction (Toxoplasma gondii)
Mechanical barriers:
-Skin and mucous membranes
•First line of defense; few organisms can penetrate intact skin unaided
•Larvae penetrate skin, go into cracks in skin, hair follicles
-Can be Zoonotic – get trapped in skin (cutaneous larva migrans)
-Can also use blood-sucking arthropod vectors to transmit disease through skin penetration;
parasite and vector work together and are both considered parasites now; I.e. mosquitoes and Dirofilaria immitis (helminth)
Chemical Barriers:
-Potent antimicrobial peptides of skin
-Sebaceous glands secrete antimicrobial sebum
•Poor/compromised immune systems - Demodex lives in sebaceous glands
-Chitin skeleton helps protect from skin secretions
-Has pin-like mouth parts for eating skin cells, hormones, and oils (sebum)
•Primarily due to hereditary deficiency in T cells
-Lysozyme – powerful enzyme in tears, saliva, other secretions
-Hydrochloric acid in the gastric mucosa
Cellular:
-Macrophages and NK cells are activated when parasite is detected but once engulfed by T cells, parasite lives and grows within cells but is not normally detected by immune system
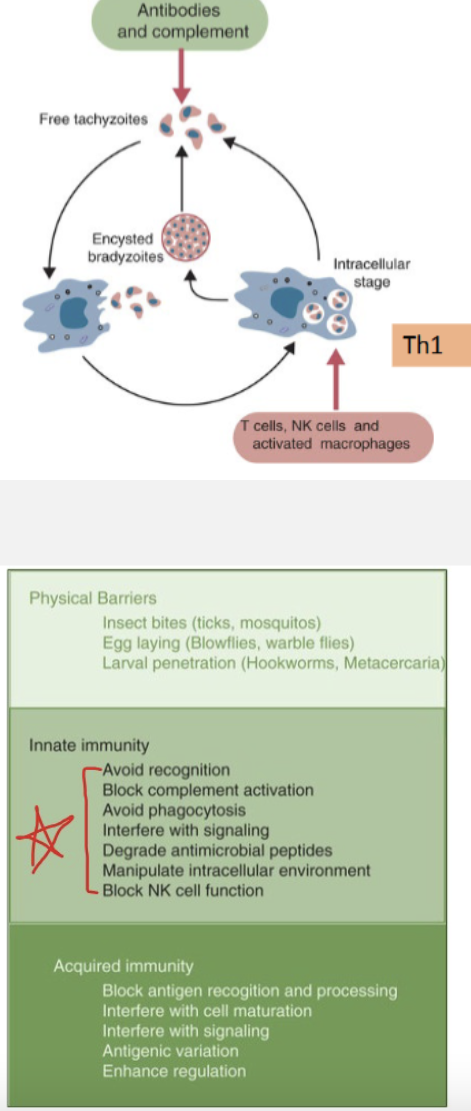
Explain how innate and adaptive responses control extra-cellular and intracellular protozoa stages, such as Toxoplasma gondii and Eimeria: complement, antibodies, macrophages, NK cells and T cells
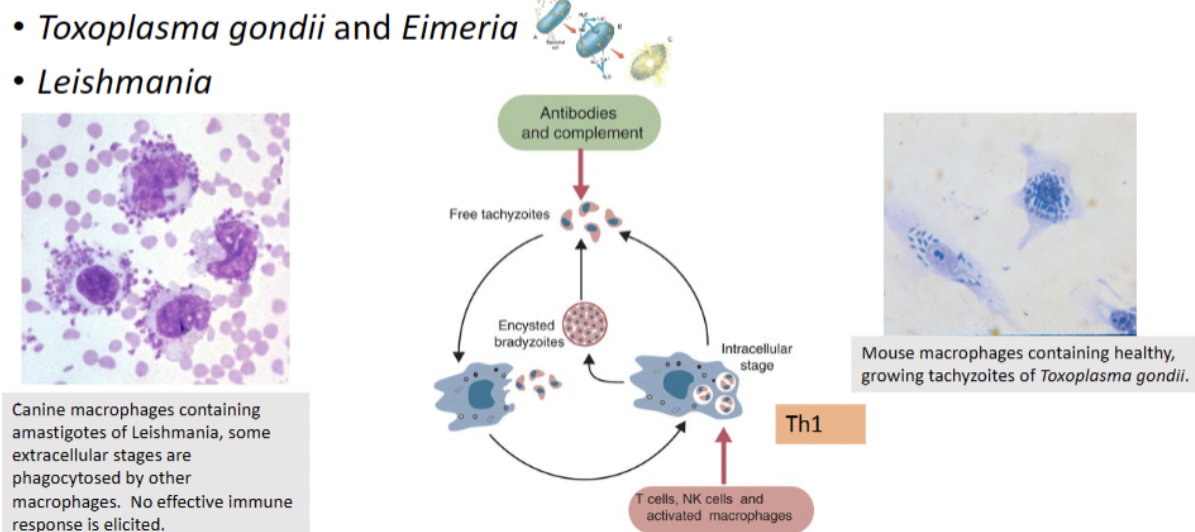
Explain parasite mechanisms (protozoa – T. gondii,Leishmania, and Trypanosomes) to either hide in the immune system or evade the immune system
-Repeated antigenic variation by the parasite
• If parasitemia is measured at regular intervals, numbers of circulating organisms fluctuate greatly – periods of high parasitemia followed by low.
• Parasites express a new surface glycoprotein antigen = Variant Surface Antigen (VSG)
• Antibodies attempt to neutralize parasites, but by the time the immune system has it figured out, the parasite changes its “coating”
• Genetically programmed (seen in tissue culture-raised trypomastigotes). Have over 1000 VSG genes. Only 1 expressed at a time
• Parasite is always one step ahead of the host. Finally host health is compromised and death occurs
Describe the immune responses to helminth parasites: granuloma, Th2 IgE response, Tuft cells, and self-cure
IgE and Granuloma:
The major Adaptive defense against gastrointestinal helminths is a Type 2 adaptive immune response.
•Antibody production of IgE (instead of IgG)
-Eosinophils and basophils
-Resembles a Type I Hypersensitivity/allergic response
•If can’t kill, develop granuloma around worm in tissue – fibroblasts develop a collagen wall = cyst.
•Cuticles cannot be penetrated by the terminal complement complex (MAC) nor by cytotoxic T cell Perforins.
TH2 helper cells produce IL-4/IL-13 which activate B cells to produce IgE
•Degranulation of Mast cells
•Produce IL-5
•Degranulation of Eosinophils
Tuft Cells and Self-Cure:
-Among the less common cells that line the small intestine are “tuft cells”, so-called because they have a small tuft of microvilli that extends into the intestinal lumen.
•Cells play a key role in initiating type 2 immune responses to helminths.
-Tuft cells apparently can sense the “taste” of intestinal helminths. In response they produce large amounts of IL-25, a cytokine that recruits eosinophils, activates both Th2 cells and ILC2 cells
•IL-25 promotes hyperplasia of tuft cells as well as mucus-producing goblet cells
•The IL-13 acts together with IL-4 to increase gut motility and mucus production.
-The combination of increased motility with increased mucus production can expel many helminth
parasites = weep and sweep phenomenon in sheep = Self cure
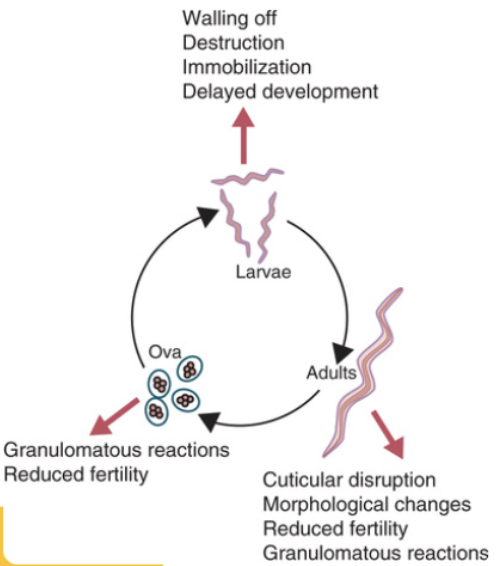
Explain the molecular basis for genetic resistance to intestinal helminths, such as Haemonchus in sheep
-Found that resistant sheep have MHC-linked genes that exert a significant influence on levels of resistance. Ability to turn-on naïve T cells.
-Found that they have accumulation of mucosal mast cells and eosinophils in the GI tract. Mechanism driven by activated T-lymphocytes (secrete IL-4 & IL-5)
List the components of tick saliva, while antigenic, still allows them to control the host’s immune system for prolonged attachment and feeding
-Tick salivary glands are not active until tick attaches. Initial and rapid synthesis of a cement protein that attaches the tick firmly to the host and seals the area around the hypostome
•Secretions seal out moisture and also act as a lubricant for the chitinous structures. Serve to recycle water from blood. Water is cycled back through the salivary glands and returned to the host
-Saliva contains digestive enzymes that assist the parasite in obtaining its blood meal. The saliva also contains components designed to minimize host responses
•Anticoagulation, antihistamine, antiplatelet, vasodilators, etc.
•Prevent host hemostatic (blood clotting) response. Host can have a Th2 response with IgE, causing inflammation which may cause tick to detach.
-Immunosuppressive and anti-inflammatory factors found in tick saliva. Collectively they act to permit prolonged tick attachment and feeding.
Explain roles of key players (interferon and NK cells) in the
innate immune response to viruses
-Innate immune response to viral infections include the induction of Type I interferons and activation of NK cells
•Distressed cells display NKG2D ligand recognized by NK cells
•Down regulate expression of MHC 1- recognized by NK cells
•Distressed cells also produce lots of type 1 interferons as a signal
Describe three mechanisms of interference with viral
replication by interferons
1.Synthesis of double stranded RNA-dependent protein kinase: inactivates translation initiation factor, eIF2α,resulting inhibition of all protein synthesis (viral and cellular)
2.Activation of latent cellular endonuclease: IFN signaling induce expression of 2’5’ OAS (oligoadenylate synthetase), which in turn activates a latent cellular ribonuclease, RNase L, which degrades mRNA
and ribosomal RNA.
3. Production of Mx proteins: Mx proteins are expressed in hepatocytes and endothelial cells inhibit viral transcription and assembly of a range of RNA viruses (Influenza).
Explain how antibodies provide immunity to viruses
Ab-mediated immunity is effective against viruses only at the extracellular stage of infection, before they enter host cells; Immunity against re-infection is mediated by circulating virus-neutralizing antibodies which prevent virus attachment and entry into host cells
•Antibodies can promote clearance of virus particles from the circulation by clumping viruses and facilitating their removal by phagocytic cells
-Agglutinate viruses by covering surface and sticking viral particles together, cannot infect cell.
-Opsonized viruses are also readily engulfed and phagocytosed.
-Lysis of some enveloped viruses by the membrane attack complex can occur when IgG or IgM bind to surface and activate complement.
•Exception to rule of virus outside of cell - Virus-infected cells ,when coated with antibody and with C3b bound to their surfaces may be engulfed and destroyed by phagocytes
Describe the role of cytotoxic T cells in immunity to viruses
-During viral infection, CD8+ T cells recognize viral antigen displayed in association with MHC class I molecules on nucleated cells and undergo rapid proliferation reaching their peak at about 10 days before declining slowly. These cells differentiate into effector cytotoxic CD8+ T cells (CTL) but require help from CD4+ TH1 cells or co-stimulation from infected cells.
-Differentiation into effector cytotoxic CD8+T cell (CTL) results in release of cytokines, IFNγ and TNF, which promote recruitment of inflammatory cells to the site of infection and interfere with viral replication
-CTLs also kill infected cells through the release of perforin and granzymes or through the induction of apoptosis by Fas/FasL interactions (death receptor).
-Most CTLs die after release of granules.
A small proportion of CTLs persist as memory cells and have distinct survival receptors
Describe mechanisms by which cytotoxic T cells kill target cells
Describe roles of different components of innate immunity in response to bacterial infection.
•TLRs are responsible in large part for the initial recognition of invading bacteria
•Binding of microbial PAMPs to TLRs triggers a signal cascade that activates cytokine genes that are critical in host defense
For example, in R. equi infection, dendritic cells and macrophages secrete IL-23, which promotes TH 17 cell differentiation
•TH 17 cells confer protection against extracellular bacteria and fungi, by triggering inflammation
•Natural Killer-NK cells play a protective role in some bacterial, protozoan and fungal infections.
•NK cells do not express antigen specific receptors-so how do they recognize altered self cells? Instead have receptors to ligands displayed by distressed cells.
For example, some bacteria cause up-regulated expression of NKG2D ligands on infected cells. Causes activation of NK cells. Activated NK cells produce a large amount of IFNγ that activate both macrophages and dendritic cells
- complement: bacteria can be destroyed by complement acting through the alternate or lectin pathways; as result these bacteria are either opsonized or lysed
-iron sequestering
-antimicrobial peptides
Describe how TH-17 plays an important role in immunity to extracellular bacteria like R. equi, and fungal pathogens
IL23 secreted by macrophages activates the Th17 cell pro-inflammatory pathway which clears the infection. On another path, INF-gamma is secreted and CD8+ T cells are activated (Th1 response) – get clearance and protective immunity. In foals where INF-gamma is deficient, get a CD4+ response (Th2 response) toward Ab production only which is not strong enough to control the infection.