BIO 311 Lecture 8: Fatty Acids and Amino Acids to Acetyl CoA
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Digestion of TAG, and roles of liver, gallbladder and pancreas in lipid digestion.
Digestion of TAG
Bile salts emulsify dietary fats in small intestine => mixed micelles
Intestinal lipases (from pancreas) degrade triacylglycerols
Fatty acids and other breakdown products are taken up by intestinal mucosa and converted into triacyclglycerols
Triacylglycerols are incorporated, w/ cholesterol and apolipoproteins, into chylomicrons
Chylomicrons move through lymphatic system and bloodstream to tissues
Lipoprotein lipase, activated by apoC-II in the capillary, converts TAG to fatty acids and glycerol.
Fatty acids enter cells
Fatty acids are oxidized as fuel or reesterified as storage
Liver: produces bile salts
Gallbladder: stores bile
Pancreas: provides intestinal lipases
Names of the abundant 12C, 14C, 16C and 18C saturated fatty acids; and the three essential fatty acids commonly present in diet.
12C
Common name: Laurate
Systematic name: n-Dodecanoate
14C
Common name: Myristate
Systematic name: n-Tetradecanoate
16C
Common name: Palmitate
Systematic name: n-Hexadecanoate
18C
Common name: Stearate
Systematic name: n-Octadecanoate
Essential fatty acids: Linoleate (18C), Linolenate (18C), Arachidonate (20C)
Lipid soluble vitamins and general understanding their roles in human biology/physiology.
Lipid soluble vitamins
G(K), D, A, E
Roles:
Vision, bone health, immune function, coagulation
Clinical consequence of malabsorption of fatty acids.
Deficiency of lipid soluble vitamins, essential fatty acids, and dehydration
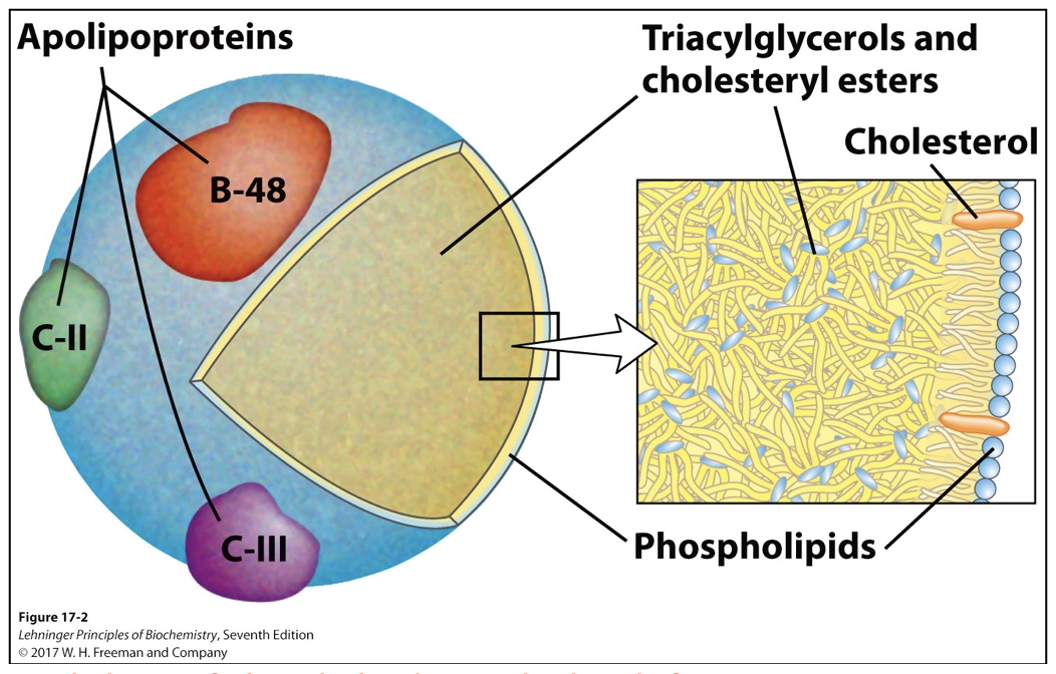
General structure of chylomicron, the role of B-48 protein.
Single layer of phospholipids -> polar heads facing water
Triacylglycerols sequestered in the interior make up more than 80% of mass
Many apolipoproteins that protrude from surface (B-48, C-III, C-II) act as signals in uptake and metabolism of chylomicron contents
Diameter ranges from about 100 to 500 nm
B-48 role: surface protein marker that identifies itself and regulates uptake
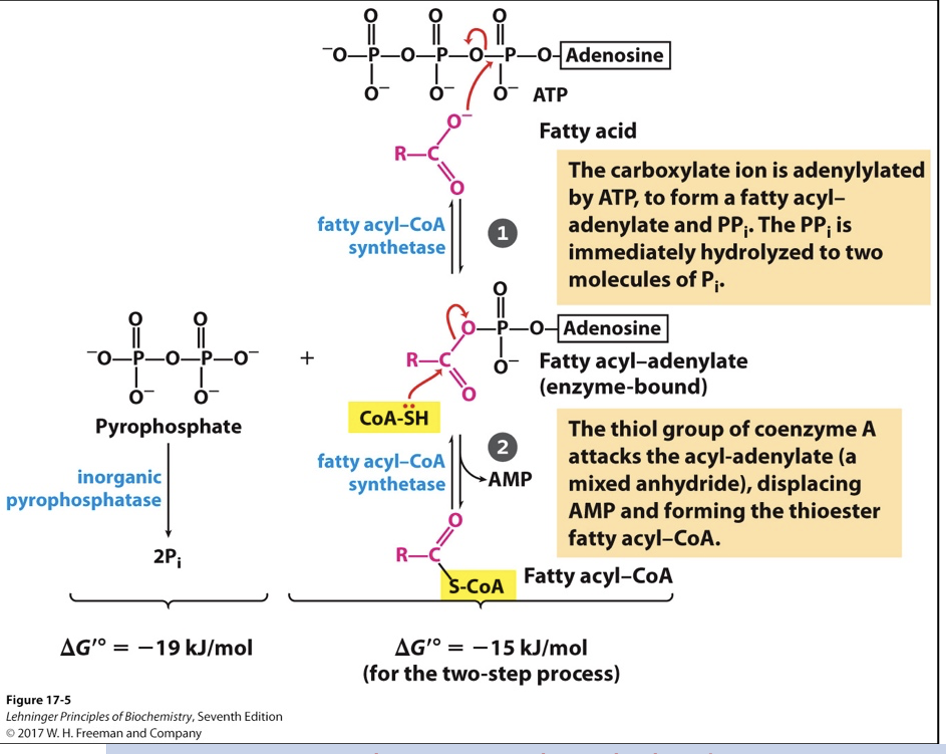
fatty acids activation in cytosol
Transport or attachment to phospholipids requires conversion to fatty acyl-CoA
Fatty acid -> Fatty acyl-CoA
Enzymes: fatty acyl-CoA synthetase and inorganic phosphatase
Highly exergonic reaction
Total: 2 ATP equivalents consumed
Consume 1 physical ATP molecule, but produce AMP
To return AMP back to ATP, use 2 inorganic phosphates (1 ATP equivalent)
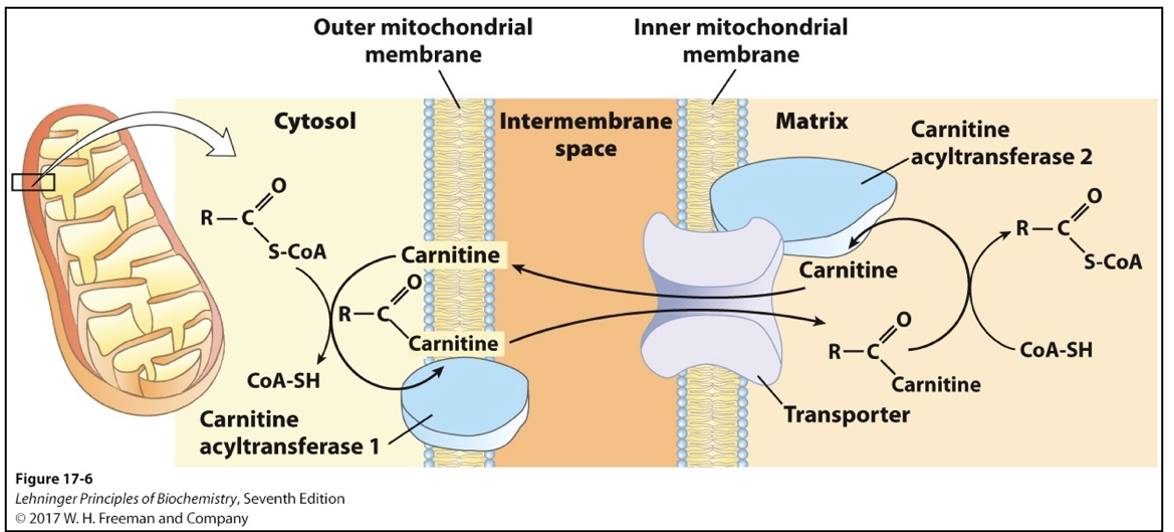
Translocation to mitochondria + Rate Limiting Step
Beta oxidation of fatty acids occurs in mitochondria
Fatty acids may be transported as free fatty acids or more commonly, by lipoproteins (chylomicrons)
Small (< 12 carbon) fatty acids diffuse freely across mitochondrial membranes
Larger fatty acids (most free fatty acids) are transported via acyl-carnitine/carnitine transporter
RATE LIMITING STEP OF FATTY ACID OXIDATION
After fatty acyl-carnitine is formed at the outer membrane or in intermembrane space, it moves into mitochondrial matrix by facilitated diffusion through the transporter in the inner membrane
In matrix, acyl group is transferred to mitochondrial coenzyme A (CoA-SH), freeing carnitine to return to intermembrane space via same transporter
Carnitine acyltransferase I (CAT I): outer mitochondrial membrane
Carnitine acyltransferase II (CAT II): inner mitochondrial membrane
Transporter: inner membrane
Carnitine
Carnitine = ID Card
Not a vitamin; synthesized by human body from lysine
Synthesis depends on vitamin C
Fatty acyl group: transferred to OH group => ester
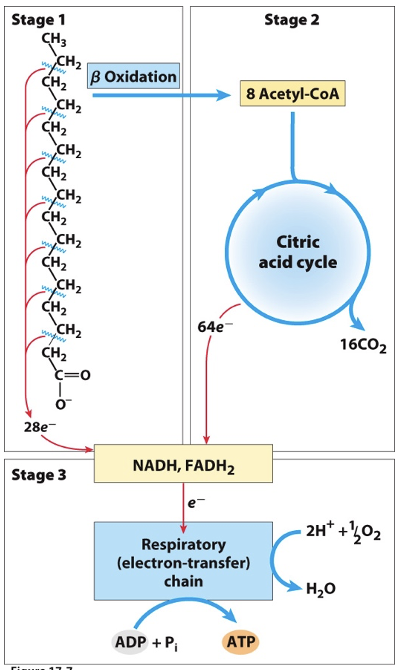
Oxidation of Fatty Acids
Stage 1 (Beta oxidation): a long-chain fatty acid is oxidized to yield acetyl residues in the form of acetyl-CoA
Stage 2: the acetyl groups are oxidized to CO2 via citric acid cycle
Stage 3: electrons derived from oxidations of stages 1 and 2 pass to O2 via mitochondrial ETC, providing energy for ATP synthesis by oxidative phosphorylation
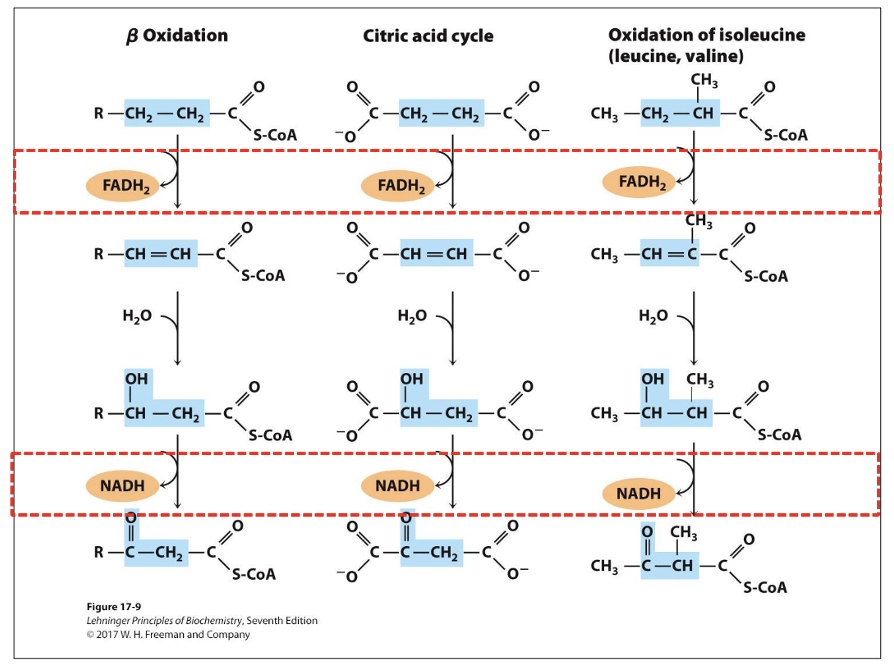
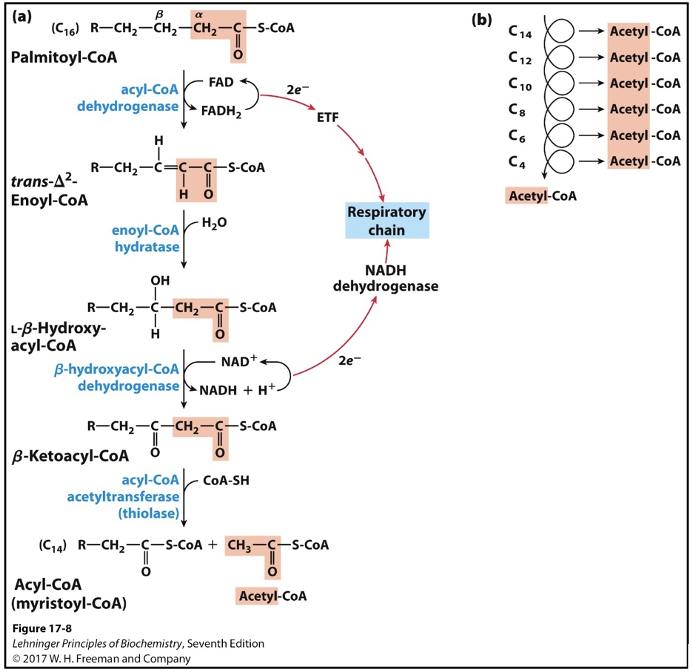
The 4 step reactions in the repeating rounds of beta-oxidation.
first three steps follows pattern similar to steps 6-8 of TCA (dehydrogenate then hydrate then dehydrogenate)
Step 1: Dehydrogenation of Alkane to Alkene
Enzyme: isoforms of acyl-CoA dehydrogenase (AD) on the inner mitochondrial membrane
FAD cofactor
Very-long-chain AD (12-18 C)
Medium-chain AD (4-14 C)
Short-chain AD (4-8 C)
Result: trans-enoyl-CoA; trans double bond, different from naturally occurring unsaturated fatty acids
Analogous to succinate dehydrogenase reaction in the citric acid cycle
Electrons from bound FAD transferred directly to the ETC via electron-transferring flavoprotein (ETF)
Step 2: Hydration of Alkene (SAYS THIS IS ENOL HOW?)
Enzyme: two isoforms of enoyl-CoA hydratase
Soluble short-chain hydratase (crotonase)
Membrane-bound long-chain hydratase, part of trifunctional complex
Result: beta-hydroxy-acyl-CoA; water adds across double bond => alcohol on beta carbon
Analogous to fumarase reaction in the citric acid cycle
Same stereospecificity
Step 3: Dehydrogenation of Alcohol
Enzyme: beta-hydroxyacyl-CoA dehydrogenase
NAD cofactor
Result: Beta-ketoacyl-CoA
Only L-isomers of hydroxyacyl CoA act as substrates
Analogous to malate dehydrogenase reaction in the citric acid cycle
Step 4: Transfer of Fatty Acid Chain and Release of Acetyl-CoA
Enzyme: acyl-CoA acetyltransferase (thiolase) via covalent mechanism
Result: thiolysis of the carbon-carbon bond
Carbonyl carbon in beta-ketoacyl-CoA is electrophilic
Active site thiolate acts as a nucleophile and releases acetyl-CoA
Terminal sulfur in CoA-SH acts as a nucleophile and picks up the fatty acid chain from the enzyme
Given an even numbered fatty acids or fatty acyl-CoA, point out the end products, including FADH2 and NADH, from beta oxidation and the amount of each.
# of FADH2/NADH2 formula (check)
(Even # / 2) – 1 = # of FADH2/NADH
Ex: Palmitic acid (16C) => 7 FADH2 and 7 NADH
Given an even numbered fatty acid or fatty acyl-CoA, if completely oxidized, the amount of ATP production with functional ETC.
# of ATP formula:
(Even # / 2) – 1) * 4 ATP = # of total ATP from beta oxidation rounds
(Even # /2) * 10 ATP = # of total ATP from ETC
Sum them up
NET ATP: Consider the two ATP reduced in cytosol
How to catabolize unsaturated and odd-numbered fatty acids? Cofactor needed for catabolism of propionyl-CoA?
Oxidation of Unsaturated Fatty Acids
We can do it!
Naturally occurring unsaturated fatty acids contain cis double bonds
NOT a substrate for enoyl-CoA hydratase
Two additional enzymes required
Isomerase: converts cis double bonds starting at carbon beta to trans double bonds
Reductase: reduces cis double bonds not at carbon beta
Monounsaturated Fatty Acids:
Require isomerase
Shift double bond, cis to trans using enoyl-CoA isomerase
1 Acyl-CoA dehydrogenase step skipped => 1 less FADH2 produced
Polyunsaturated Fatty Acids:
Require both enzymes (isomerase and reductase)
We can do it!
Less energy yield
Involves NADPH dependent reductase
Oxidation of Odd-Numbered Fatty Acids
We can do it!
Most dietary fatty acids are even-numbered (b/c of fatty acid synthesis), but many plants and some marine organisms synthesize odd-numbered fatty acids => our diets
Propionyl-CoA: 3 carbon compound that forms during final cycle of beta oxidation of odd-numbered fatty acids
Some amino acids also produce propionyl-CoA
Oxidation of Propionyl-CoA pathway:
When beta oxidizing odd-numbered fatty acids, you eventually get to propionyl-CoA
Propionyl-CoA -> D-Methylmalonyl-CoA
Enzyme: propionyl-CoA carboxylase
Cofactor: biotin
D-Methylmalonyl-CoA -> L-Methylmalonyl-CoA
Enzyme: methylmalonyl-CoA epimerase
L-Methylmalonyl-CoA -> Succinyl-CoA
Enzyme: Methylmalonyl-CoA mutase
Cofactor: Coenzyme B12 (cobalamin)
Send to TCA Cycle
VITAMIN B12 DEPENDENT
Most complicated B family vitamin
Cobalt ion in the middle
B12 is synthesized by microbials (need animal source diet -> egg, fish, etc.)
Consequence of defective medium chain fatty acyl CoA dehydrogenase.
1/10000 births
Defects in fatty acids utilization
Causes hypoglycemia
Causes sudden infant death syndrome (10% of cases)
Roles of stomach and pancreas in protein digestion and three types of enzymes involved in protein digestion.
Protein digestion start in stomach via pepsin enzymes => cleaves peptide bonds, reducing large polypeptides to smaller polypeptides
Small polypeptides move to small intestine via peristalsis, where proteases (synthesized in enzyme) break them down into short polypeptides (tripeptides, dipeptides), and individual amino acids
Enzymes in small intestine epithelium eventually break the short polypeptides into amino acid monomers
General pattern of amino acids catabolism to form metabolites of Krebs cycle
Removal of Amino group from amino acid => alpha-keto acid ; transfer amino group to alpha-ketoglutarate => glutamate
Fates of alpha-keto acid x:
Directly a metabolite of TCA cycle
ndirectly converted to a metabolite of TCA cycle
If generate ketone body or Acetyl-CoA: ketogenic
If not: glucogenic (important in carbohydrate metabolism)
Catabolism of one amino acid may synthesize another one
How to remove non-alpha amino/amine groups?
Majority: Alpha amino groups -> Transamination
Amino group from alpha-amino acid transferred to alpha-ketoglutarate to form alpha-keto acid X and glutamate, respectively
Other:
Non-alpha amine (glutamine, asparagine, arginine) -> Hydrolysis
-OH groups (Serine, threonine) -> Dehydration-Initiated Deamination
Glutamate/Glutamic acid in Urea Cycle -> Oxidative Deamination
Other pathways to TCA

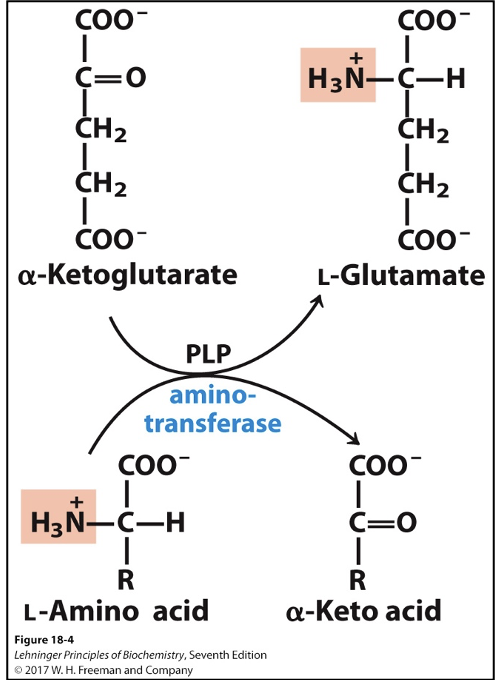
Transamination reaction, aminotransferase and cofactor needed. Vitamin precursor?
Enzyme: aminotransferase
Cofactor: PLP (pyridoxal phosphate)
Precursor: vitamin B6
Reversible reaction
Involves either:
Alpha-ketoglutarate (amino acceptor)
Glutamate (donor)
Things to know about vitamin B6
Transamination always needs vitamin B6
Amino acid metabolism (ex: decarboxylation of amino acid) needs vitamin B6
Vitamin B6 participates in various reactions
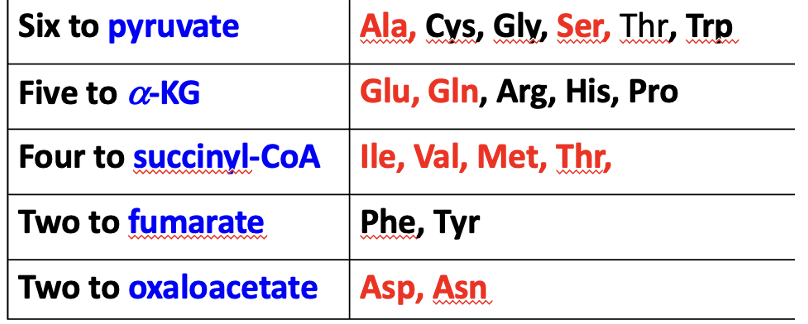
Overall correlation between common organic compounds including ketoacids (2C-5C) and corresponding amino acids precursors.
2C: Acetyl-CoA (ketogenic -> you do not increase carbons in TCA cycle -> not glucogenic -> not anaplerotic)
3C: Propionyl-CoA (3C) to Succinyl-CoA (glucogenic)
4C: Oxaloacetate (glucogenic)
5C: Alpha-Ketoglutarate (glucogenic)
The conserved pattern of oxidation of reduced carbon molecules.
TCA cycle, beta-oxidation, and amino acid oxidation follow same pattern same sequence: DEHYDROGENATION -> HYDRATION -> DEHYDROGENATION
Dehydrogenation b/t alpha-beta generate double bond and FADH2
After those common steps, structure differences determine further steps of metabolism.