3. CNS 4 Inhibition in the Brain
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
What is GABA & how is it synthesized?
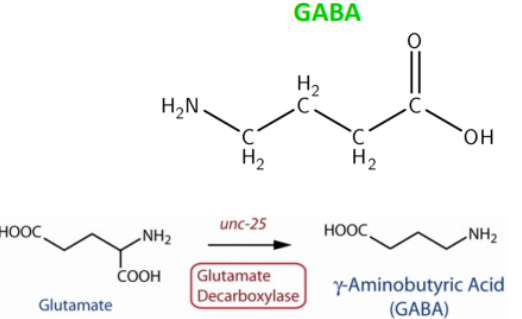
Most abundant inhibitory neurotransmitter in the brain
Synthesized from glutamate via glutamic acid decarboxylase (GAD)
Neurones that synthesise GABA are called inhibitory GABAergic neurones
Acts at inhibitory synapses to suppress neuronal activity
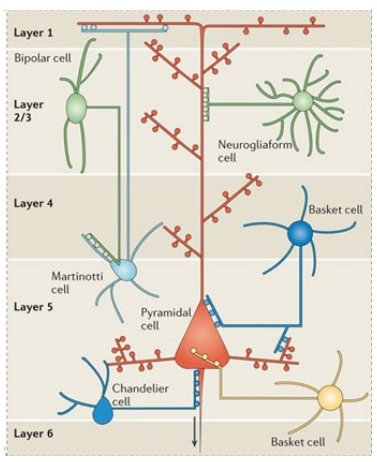
What are the two main types of inhibitory GABAergic neurones in the brain?
Interneurones
Innervate nearby neurones (excitatory pyramidal or other inhibitory interneurones)
Control activity of large groups of neurones via widespread synapses
Mediate strong synchronisation of activity
~20 different types with varied morphology & brain location
Projection neurones
Innervate neurones outside their region (e.g. medium spiny neurones of striatum)
How does GABA cause inhibition at the synapse?
GABA is stored in vesicles in presynaptic terminal
AP arrival → vesicle fusion with membrane → GABA released into synaptic cleft
GABA binds to GABA receptors (Cl⁻ channels) on postsynaptic membrane
Cl⁻ flows into cell (high [Cl⁻] outside → low [Cl⁻] inside)
Influx of negatively charged Cl⁻ → membrane becomes more negative (hyperpolarisation)
This is called an inhibitory postsynaptic potential (IPSP)
Hyperpolarisation ↓ probability of excitation by making it harder to reach threshold
If excitatory & inhibitory inputs occur together, they may cancel out → no net change
![<ul><li><p class="">GABA is stored in vesicles in presynaptic terminal</p></li><li><p class="">AP arrival → vesicle fusion with membrane → GABA released into synaptic cleft</p></li><li><p class="">GABA binds to GABA receptors (Cl⁻ channels) on postsynaptic membrane</p></li><li><p class="">Cl⁻ flows into cell (high [Cl⁻] outside → low [Cl⁻] inside)</p></li><li><p class="">Influx of negatively charged Cl⁻ → membrane becomes more negative (hyperpolarisation)</p></li><li><p class="">This is called an inhibitory postsynaptic potential (IPSP)</p></li><li><p class="">Hyperpolarisation ↓ probability of excitation by making it harder to reach threshold</p></li><li><p class="">If excitatory & inhibitory inputs occur together, they may cancel out → no net change</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/fb7e7fa9-5707-47a7-a45c-e94e672d8779.png)
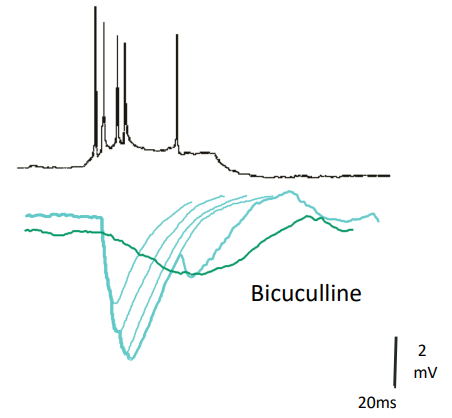
How are IPSPs recorded & what does bicuculline show?
Presynaptic GABAergic neurone fires APs → causes IPSPs in postsynaptic neurone
IPSPs = small transient hyperpolarisations
Bicuculline (GABAA antagonist) blocks fast IPSPs
Blocking GABAA reveals slower GABAB-mediated inhibition
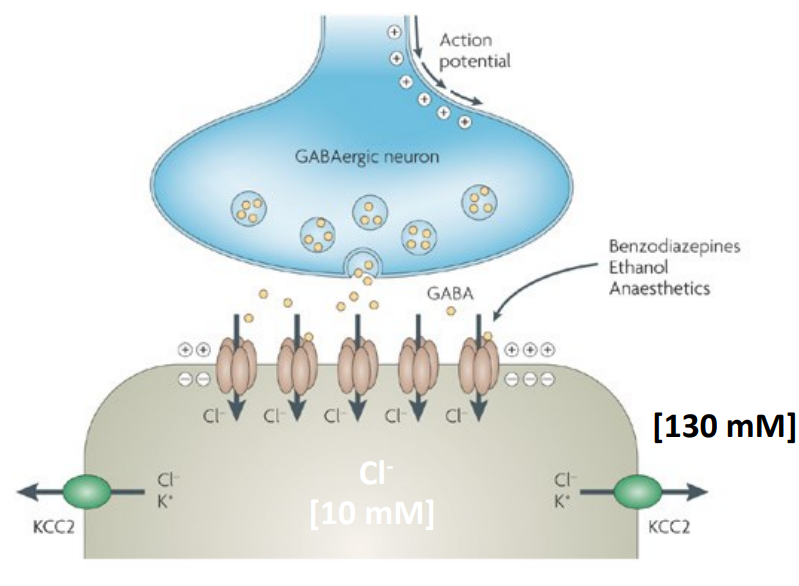
How does KCC2 maintain GABAergic inhibition in the brain?
KCC2 pumps Cl⁻ out of the cell, maintaining low intracellular Cl⁻
This creates a Cl⁻ gradient that allows Cl⁻ to enter via GABAA receptors
Cl⁻ influx causes hyperpolarisation = inhibition
Without KCC2, Cl⁻ builds up = ↓ inhibition = risk of seizures & death
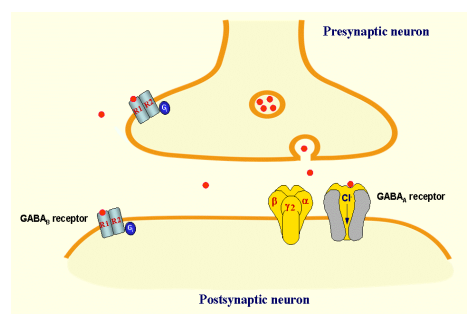
What are the two types of GABA receptors & how do they function?
Ionotropic GABA receptors:
Ligand-gated ion channels
GABA binding opens channel → ion influx (e.g. Cl⁻)
Metabotropic GABA receptors:
G-protein coupled receptors
GABA binding → activates G-proteins & intracellular signalling (= longer-lasting inhibition of postsynaptic neurones
Both types can be presynaptic or postsynaptic
What are the main types & functions of ionotropic GABA receptors?
GABAA receptors: found in CNS
GABAC receptors: found in retina
Mediate Cl⁻ influx (minor HCO₃⁻ outflow)
Cause membrane hyperpolarisation = inhibition of EPSPs
GABAA agonists/allosteric modulators ↓ seizures & GABAA antagonists ↑ seizures
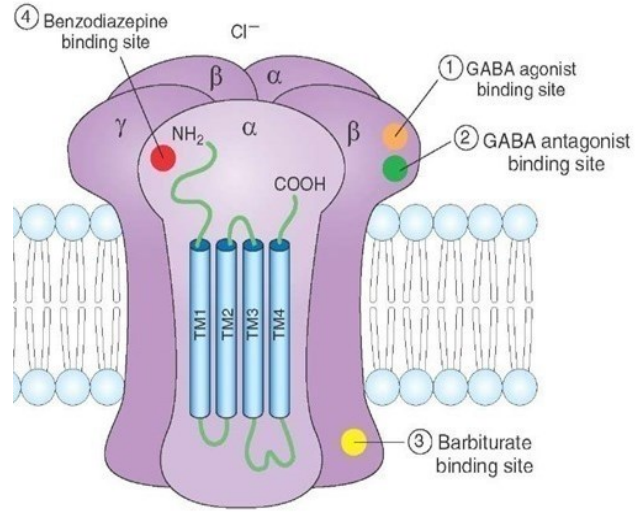
What are key features of GABAA receptors?
Expressed in all brain neurons
Involved in anxiety, epilepsy, panic disorders & insomnia
Targeted by benzodiazepines, barbiturates, anaesthetics & alcohol
Modulated by stress hormones & neurosteroids
Pentameric ligand-gated ion channels
Pentameric: 2 α, 2 β, 1 γ/δ/ε/π/θ subunit
GABA binds at α-β interface
Benzodiazepines bind at α-γ interface
Barbiturates bind intracellularly
Subunit composition affects:
GABA affinity
Channel properties
Drug sensitivity
Cell type-specific expression
Subcellular localisation (e.g., γ needed for synaptic, δ for extrasynaptic → tonic inhibition)
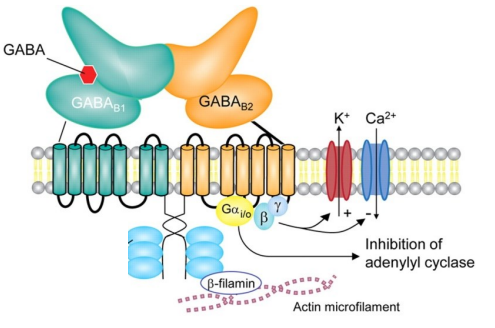
How do GABAᵦ receptors function & what are their effects?
GABAᵦ receptors are metabotropic, G-protein coupled (R1 & R2 subunits)
Agonist = baclofen
Gαᵢ/ₒ subunit = inhibits adenylyl cyclase = ↓ protein kinase A (PKA) activation
PKA = enzyme that phosphorylates proteins to regulate cell activity
β & γ subunits:
Activate K⁺ channels = hyperpolarisation
Inhibit Ca²⁺ channels = ↓ neurotransmitter release
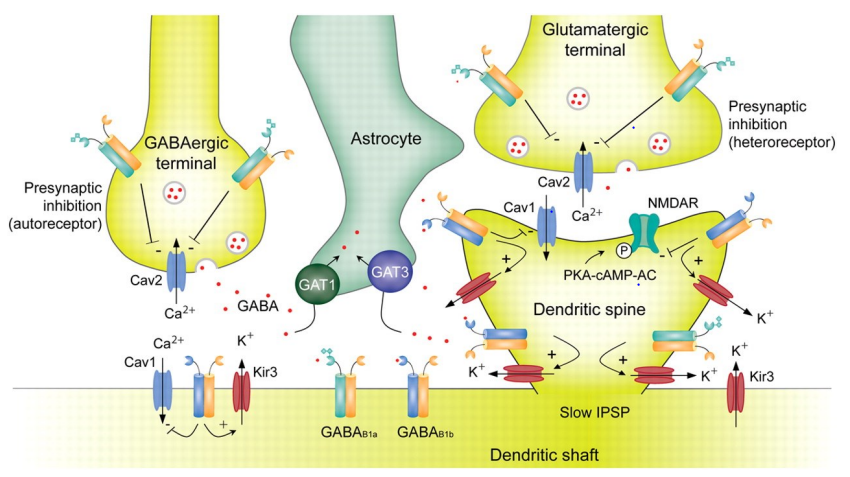
What does the diagram show about GABAᴮ receptor localisation & function at synapses?
GABAᴮ receptors act at both pre- & postsynaptic sites, with different effects depending on location & cell type
Presynaptic (autoreceptor) on GABAergic terminal:
Activated by GABA it releases itself
Inhibits Cav2 Ca²⁺ channels = ↓ Ca²⁺ influx = ↓ GABA release
Presynaptic (heteroreceptor) on glutamatergic terminal:
Activated by GABA from nearby GABAergic terminal
Inhibits Cav2 channels = ↓ Ca²⁺ influx = ↓ glutamate release
Postsynaptic (on dendritic shaft/spine):
Activates Kir3 K⁺ channels = K⁺ efflux = hyperpolarisation
Produces a slow IPSP
Also: Gαᵢ/o inhibits adenylyl cyclase = ↓ cAMP = ↓ PKA activation
Astrocytes (GAT1 & GAT3) remove excess GABA
Net effect depends on receptor location (pre/post) & cell type (GABAergic/glutamatergic)
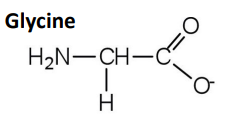
What are the key features of glycine as an inhibitory neurotransmitter?
Main inhibitory neurotransmitter in spinal cord & brainstem
Glycine = simple amino acid
Glycine receptors (GlyRs) = ligand-gated Cl⁻ channels with α & β subunits
Activation = Cl⁻ influx = hyperpolarisation of postsynaptic membrane = ↓ neuronal firing
Blocked by strychnine (competitive antagonist) = overexcitation: pain, cramps, startle response
Also mediates inhibition in retina via glycinergic amacrine cells