Atomic Theory
1/139
Earn XP
Description and Tags
credit to Nevan P; this is only a combined set of their flashcards + three extra self made
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
140 Terms
Thomson
His model was the first to include negative particles (electrons)
Bohr
His model was the first that said electrons are organized in energy levels. Also, they can lose or gain specific amounts of energy to move from level to level.
Schrodinger
His model refers to probable electron locations as orbitals or clouds
Dalton
Atoms are indivisible, solid spheres
Rutherford
Gold Foil Experiment
Thomson
Did experiments with cathode ray tubes
Dalton
His theory did not include any subatomic particles
Thomson
Plum pudding model
Rutherford
First to conclude that all mass and positive charge is located in a very small center (nucleus)
Thomson
Visualized the atom as a solid + body with electrons embedded in the sphere
Bohr
Visualized electrons moving in concentric, circular orbits (energy levels) around the nucleus
Dalton
His early theory said all atoms of an element are identical and different from atoms of other elements; atoms do not change during chemical reactions - they just rearrange
Rutherford
First to conclude that atom is mostly empty space
Thomson
His model is called the "plum pudding" model
Schrodinger
His model is nicknamed the "quantum mechanical" model and based on complex mathematical equations.
Thomson
Discovered the first subatomic particle
Dalton's Atomic Theory (1808)
- Each element is made up of tiny particles called atoms
- The atoms of a given element are identical
- Chemical compounds are formed when atoms of different elements combine (chemical reaction)
- Chemical reactions involve reorganization of the atoms
Thomson's Atomic Theory: Plum Pudding Model (1904)
- Atoms are made of a positively charged cloud (negative electrons are embedded)
- Atoms are spheres of positively charged "dough" or fluid with negatively charged electrons (the "plums" or "raisins") scattered throughout, creating a neutral atom overall
- The total positive charge of the sphere balanced the total negative charge of the scattered electrons, making the atom electrically neutral.
Rutherford Atomic Theory: The Nuclear Atom (1911)
- Atoms have a dense, positively charged nucleus at its center containing most of the atom's mass, surrounded by a large amount of empty space where negatively charged electrons orbit.
- Nucleus: A tiny, dense, positively charged center of the atom that contains protons and almost all the atom's mass.
- Electrons: Light, negatively charged particles that revolve around the nucleus at some distance.
- Empty Space: The vast majority of the atom's volume consists of empty space.
The Modern View
- Composition of an atom:
- Protons
- Found in the nucleus
- Positive charge
- Electrons
- Found outside the nucleus
- Negative charge
- Neutrons
- Found in the nucleus
- No charge
- Describes an atom as having a central, dense nucleus containing positively charged protons and neutral neutrons, surrounded by a diffuse electron cloud of negatively charged electrons
Atom
Smallest particle of an element
Molecule
Two or more atoms joined together acting as a unit
Ions
positively (cation) and negatively (anion) charged atoms
Isotopes
Atoms with the same number of protons but different numbers of neutrons
Atomic Radius
The measurement of an atom's size, defined as the distance from its nucleus to its outermost electron shell
- Decreases as it goes across a period from left to right (Electrons are drawn closer to the nucleus --> size decreases)
- Increases when going down in a group (Orbital size increases in a group going from top to bottom)
Natural Law
- A fundamental chemical law
- An observation of mass that applies to many different systems
Law of Conservation of Mass
- Stated by Antonio Lavoisier
- Mass is neither created or destroyed
Law of definite Proportion
- Stated by Joseph Proust
- A given compound always contains the same proportion of element by mass
Ionization of Energy
The amount of energy required to remove an electron from an atom/gaseous state
- While going across a period from left to right, the ionization energy increases
- While going down a group from top to bottom, the energy decreases
Electron Affinity
the energy change associated with the addition of an electron to a gaseous atom or ion
- he process can be represented as: X(g) + e⁻ → X⁻ + energy
The Atomic Model of Bohr (Orbit)
The electron in a hydrogen atom moves around the nucleus in a certain allowed circular orbits.
Ground state: The most stable configuration of the Orbit (Orbit 1 closes to the nucleus)
Drawbacks:
- The model can only be applied to hydrogen atoms
- The electrons do not move around the nucleus in circular orbits
Constants:
- Planck's Constant: 6.63 x 10^-34 j/s
- Speed of light: 3x10^8 m/s
Quantum Mechanical Model
The region of the atom where the probability to find an electron is higher.
S, p, d, f
Quantum Numbers
They express the properties of the orbital
Principal Quantum Number (N)
- Related to the size and energy of the orbital
- 1, 2, 3, 4, 5...
Angular Quantum Number (L)
- Related to the shape
- 0, 1, 2, 3, 4
Magnetic Quantum Number (Ml)
- Related to the orientation
- 1, 0, -1...
Electron spin quantum number (Ms)
- Electrons can spin in either of two possible directions
- +1/2 or -1/2
Heisenberg uncertainty principle
If we know the speed of an electron, we cannot know the position and vice versa.
Pauli Exclusion Principle
In a given atom, no two electrons can have the same set of quantum numbers.
C (speed of light)
3×10^8 m/s
h (Planck’s Constant)
6.63×10^-34 js
Formula for wavelength
λ = hc/E
Formula required to excite the hydrogen electron
E= -2.17×10^-18(Z²/n²) —> ΔE = E_final - E_initial (z= nuclear charge nitrogen is 1 so z is always 1—-n= Orbit *the orbit closet to the nucleus in 1 and everything after that goes in numerical order)
Formula for frequency
f = c/λ
Polyelectronic Atoms: Aufbau Principle
Atoms with more than one electron
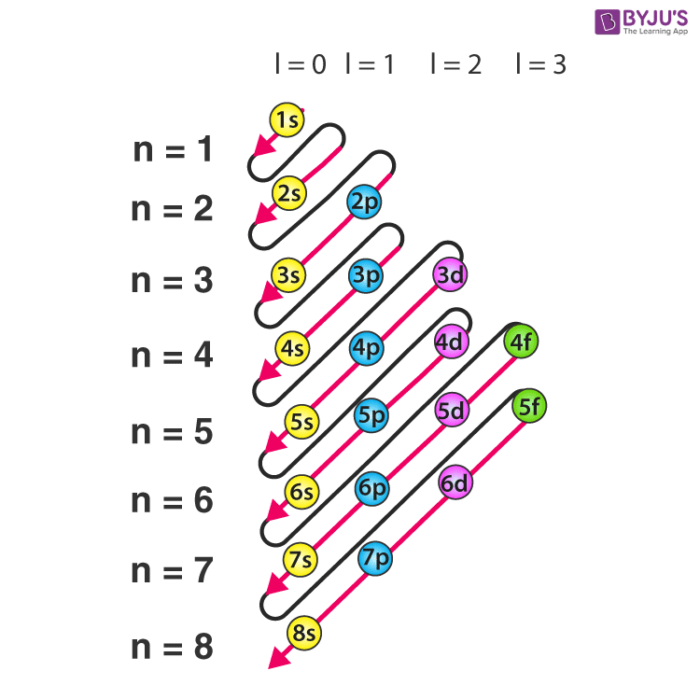
Electronic configuration
Orbitals: Quantum Number Values
s —> 2e- L= 0 s= 1
p —> 6e- L= 1 p= 3
d —> 10e- L= 2 d= 5
f —> 14e- L= 3 f= 7
Niels Bohr
Who made the Planetary Model?
Ernest Rutherford
Who created the nuclear model?
J.J. Tomson
Who created the Plum Pudding model?
Erwin Schrodinger
Who created the Quantum Model?
John Dalton
Who created the Solid Sphere Model
No nucleus, didn't explain later experimental observations.
Plum Pudding Model's problem
None according to the card sort.
Quantum Model problem
Atoms aren't indivisible-they're composed from subatomic particles
Solid Sphere model problem?
Moving electrons should emit energy and collapse into the nucleus; model did not work well for heavier atoms.
Planetary Model problem
What is the most accurate model of the atom?
Quantum Model
What is the pro of the Quantum model?
Electrons do not move in orbits; their position is uncertain.
Solid Sphere Model
Dalton drew upon the Ancient Greek idea of atoms (the word 'atom' comes from the Greek 'atomos' meaning indivisible). His theory stated that atoms are indivisible, those of a given element are identical, and compounds are combinations of different types of atoms.
Plum Pudding Model
Thomson discovered electrons (which he called 'corpuscles') in atoms in 1897, for which he won a Nobel Prize. He subsequently producted the 'plum pudding' model of the atom. It shows the atom as composed of electrons scattered throughout a spherical cloud of positive charge.
Planetary Model
Bohr modified Rutherford's model of the atom by sitting that electrons moved around the nucleus in orbits of fixed sizes and energies. Electron energy in this model was quantized; electrons could not occupy values of energy between the fixed energy levels.
Quantum Model
Schrodinger states that electrons do not move in set paths around the nucleus, but in waves. It is impossible to know the exact location of the electrons; instead, we have 'clouds of probability' called orbitals, in which we are more likley to find an electron.
Democritus - 1st
atoms are the smallest unit of matter - false —> subatomic paticles
atoms are indivisible (unable to be divided or separated)
John Dalton - 2nd
Atoms are indestructible, indivisible spheres
atoms of the same element are identical
“Billiard ball model“ —> an atom in a solid, uniform sphere (no distinguishable parts)
Cathode ray experiments
high voltage connected to terminals in a vacuum tube
rays emanated from the negative electrode (cathode) attracted to the positive plate
since the rays were the same no matter the metal this proved that electrons are the negatively charged particles in atoms
J.J Thomson
J.J Thomson - 3rd
“Plum Pudding“
an atom is a positively charged mass with discrete negative particles throughout
Ernest Rutherford - 4th
“Gold foil experiment“
“Nuclear model”
Gold foil experiment
positively charged alpha (a) particles (type of radiation) were shot at a very thin sheet foil
most particles went straight through, but some particles were deflected and a few even bounced back
the “nuclear“ model
atoms are mostly empty space with a small, dense, positively charged nucleus
electrons move in the empty space that makes up the rest of the atom
limitations - Ernest Rutherford
nucleus composed f positive charges should break apart die to repulsion force
could not explain total mass of an atom (missing the neutrons)
moving charges usually give off energy so electrons should lose energy and crash into the nucleus but this doesn’t happen
electromagnetic radiation
light is a form of energy called electromagnetic radiation
Visible light is a very narrow region of the EM spectrum
Wavelength
the shortest distance between equivalent points on a continuos wave
peak to peak or trough to trough
measured in nanometers (1nm = 1 × 10^-9 m)
frequency (v)
refers to the number of complete wave cycles that pass a given point in a unit of time
1 Hz represents 1 cycle per second
photons
discrete packets of energy
photon energy is directly proportional to frequency and inversely proportional to wavelength
electromagnetic radiation
highest energy : Gamma rays, x rays, Ultraviolet light, visible light, infrared light, microwaves, radio waves : lowest energy
short wavelengths = high frequency, high energy
long wavelengths = low frequency, low energy
Neils Bohr - 5th
spectral line experiment
Bohr’s atomic model
Quantized energy
Spectral line experiment
high voltage connected to terminal tubes containing hydrogen gas
when electricity hits an electron it moves further away from the nucleus - “excited state”
when an electron spontaneously drops down to its ground state it gives off an amount of energy which can sometimes be seen as colour of visible light
Prism
when emitted through a prism only four distinct colours of light (line spectrum) were seen
red, aqua, blue and purple
Bohrs atomic model
positively charged nucleus with electrons travelling around it in specific energy levels
Quantized energy
because is restricted to only certain discrete quantities
-specific amount of energy
Bohrs limitations
his theory could only explain line spectrum of hydrogen of hydrogen could NOT accurately predict the spectral lines of multi-electron atoms
James chadwick - 6th
discovered neurons - keeps the nucleus stable
subatomic particles
protons - identity of an element - never change
electrons - influence reactivity of an element → noble gases = stable
Neutrons - lend stability to the nucleus → holds protons together
Quantum Mechanics
the application of quantum theory to explain the properties of matter, particularly electrons in atoms
Louis de Broglie
Einstein theorized that light is both a wave and a particle
used that theory and Planck constant to say that if a wave could behave like a particle then a particle could behave like a wave
“electrons have a wave nature“
Erwin Schrodinger
electron bound to the nucleus of an atom resembles a standing wave
the circular standing wavelengths are multiples of whole numbers
only certain circular orbits have a circumference into which a whole number of wavelengths can fit
Heisenberg Uncertainty Principle
It is impossible to simultaneously know the exact position and momentum of a particle
you can only know one or the other at any given instant
since and electron is so small its hard to be precise
Wave functions
contain three variables called quantum numbers
putting specific combinations of these quantum numbers into the wave equation produce a region of space around the nucleus where an electron is found - orbital
Orbital
used to describe this region of space where electrons may be found
electron probability density
mathematical or graphical representation of the chance of finding an electron in a given space no clear boundaries = “fuzzy clouds“
higher density = higher probability of finding an electron
“boundaries“ region in which the electron spends 90% of its time
Quantum numbers
first three describe the distribution of electrons in the atom the fourth describes the behavior of each electron
n, L, Ml, Ms
n
energy level or shell of an atomic orbital and its relative size
L
Orbital shape - refers to the energy sublevels within each principle energy level
values of L are dependent on n →n-1 = L
L = 0 = s
L = 1 = p
L = 2 = d
L = 3 = f
sublevels increase in energy from s to f
ml
magnetic quantum number
describes the orientation of the orbital in the space around the nucleus
whole number values between +L -L
the number of different values = number of orbitals
ms
the sin quantum number
describes the spin of an electron
only be two values
+1/2 , -1/2
Each orbital can hold a maximum of 2 e-
s sublevel →2 electrons in it
p sublevel →6 electrons in it
d sublevel →10 electrons in it
f sublevel →14 electrons in it
s orbital
sphere
1 orbital
p orbital
peanut
x y z axis
3 orbitals
d orbital
dragonfly
5 orbitals
f orbital
flower
7 orbitals