AQA A level Chemistry 3.3.9: Carboxylic acids
1/112
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
113 Terms
What is the functional group of carboxylic acids? (1)
The functional group of carboxylic acids is -COOH
How are carboxylic acids made? (2)
1. Carboxylic acids are made by oxidising a primary alcohol to an aldehyde.
2. It is then further oxidised to form the carboxylic acid
Write the general reaction for the oxidation of a primary alcohol to an aldehyde. (1)
RCH2OH + [O] → RCHO + H2O
Provide an example of a primary alcohol being oxidized to an aldehyde. (1)
CH3CH2OH + [O] → CH3CHO + H2O
Write the general reaction for the oxidation of an aldehyde to a carboxylic acid. (1)
RCHO + [O] → RCOOH
Provide an example of an aldehyde being oxidised to a carboxylic acid. (1)
CH3CHO + [O] → CH3COOH
Write the overall reaction for the formation of a carboxylic acid from a primary alcohol. (1)
CH3CH2OH + 2[O] → CH3COOH + H2O
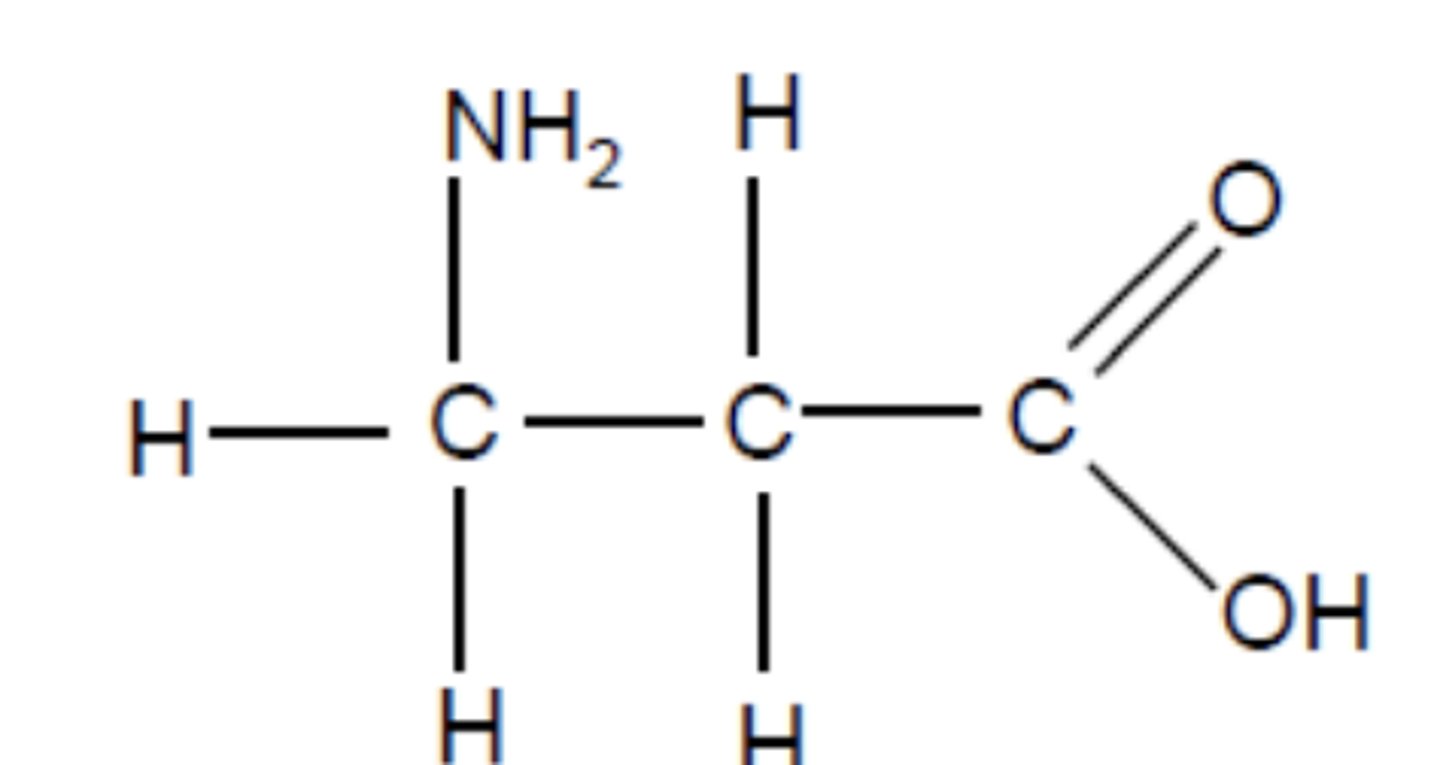
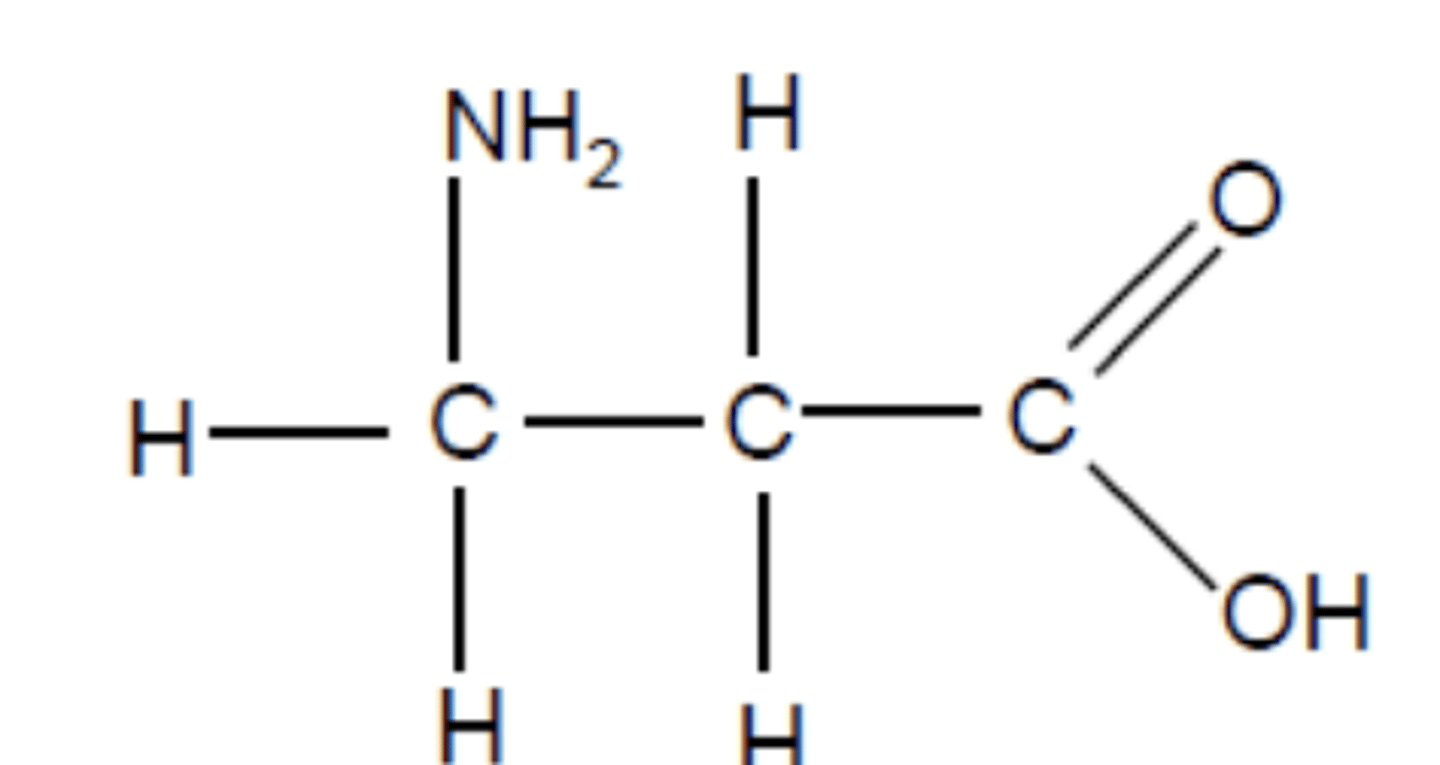
Draw the structure of 3-aminopropanoic acid (3)
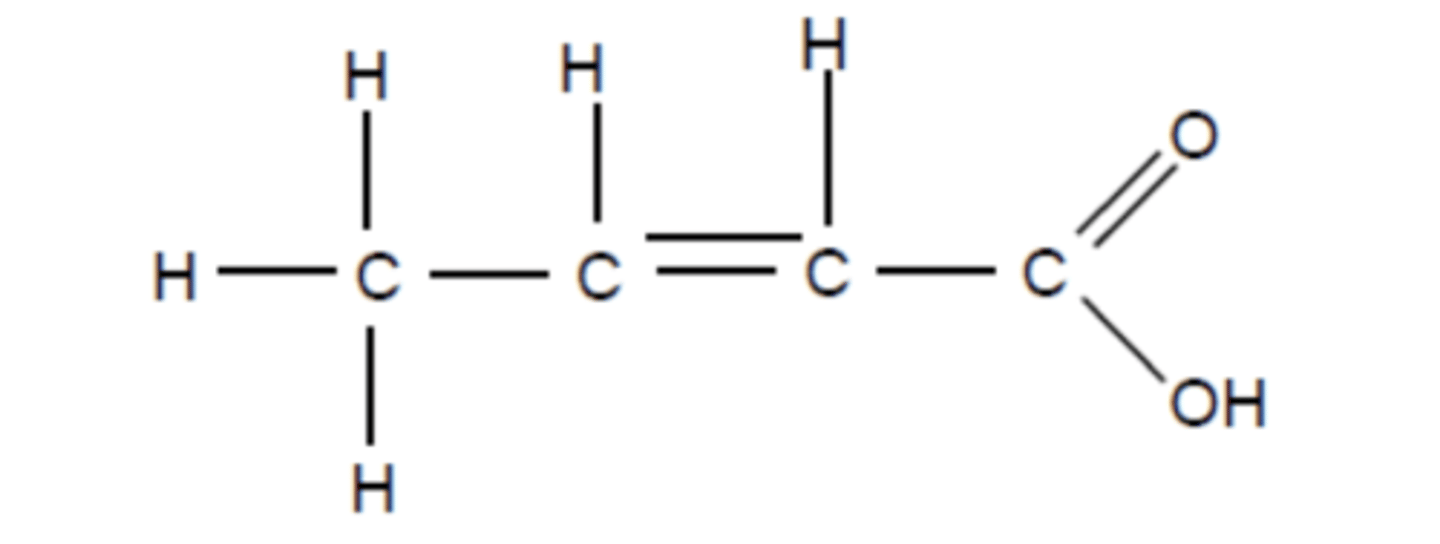
Draw the structure of but-2-enoic acid (3)
Why are carboxylic acids with a low molecular mass very soluble in water? (1)
The -COOH group forms hydrogen bonding with water
Why are carboxylic acids considered weak acids despite being very soluble in water? (1)
They are only slightly dissociated in water
Write the dissociation equation for ethanoic acid in water. (1)
CH3COOH (aq) ⇌ CH3COO⁻ (aq) + H⁺ (aq)
What is the role of the carbonyl group in the dissociation of a carboxylic acid in water? (2)
- The carbonyl group attracts electrons away from the -OH group
- Weakening the bond and allowing it to release a proton
How does the addition of a halogen atom, such as chlorine, affect the acidity of a carboxylic acid? (3)
- The halogen atom withdraws electron density away from the carbonyl carbon
- Making the O-H bond more polar and easier to break.
- This increases the acid's ability to release a hydrogen ion
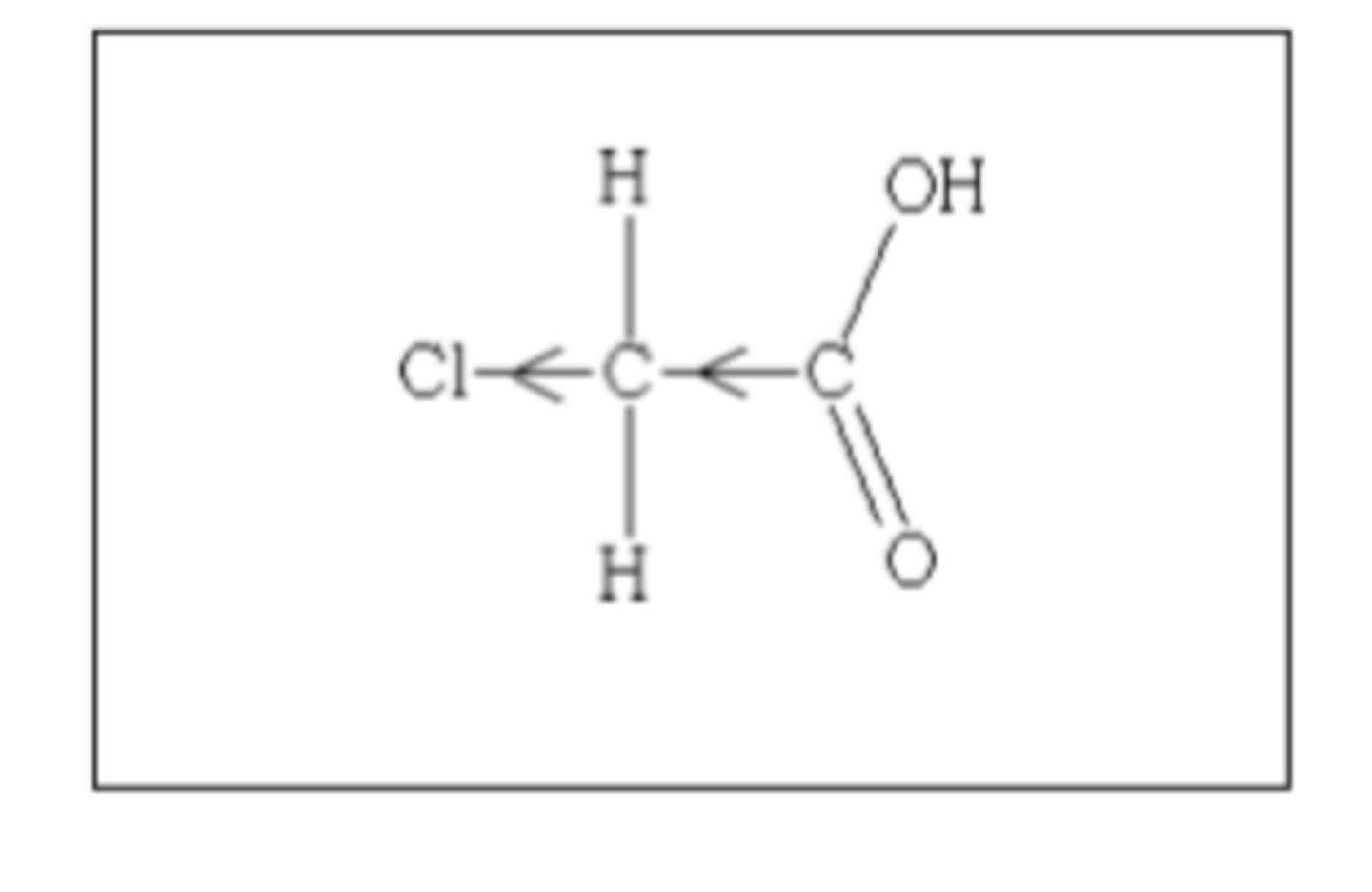
Draw the structure of chloroethanoic acid (2)
How can you test for carboxylic acids using sodium carbonate? (2)
1. React the carboxylic acid with sodium carbonate (Na₂CO₃)
2. Effervescence occurs due to the evolution of carbon dioxide gas
Write the balanced equation for the reaction of ethanoic acid with sodium carbonate. (1)
2CH₃COOH + Na₂CO₃ → 2CH₃COO⁻Na⁺ + CO₂ + H₂O
Why are ionic salts formed in the reaction between carboxylic acids and sodium carbonate? (2)
1. The reaction forms carboxylate salts
2. Which are ionic and soluble in water.
What is the reaction called when carboxylic acids react with alcohols to form esters? (1)
Esterification
What are the products of an esterification reaction? (1)
An ester and water
What catalyst is used in the esterification reaction? (1)
Concentrated sulfuric acid (H₂SO₄)
Give the general reaction equation for esterification. (1)
RCOOH + R'OH ⇌ RCOOR' + H₂O
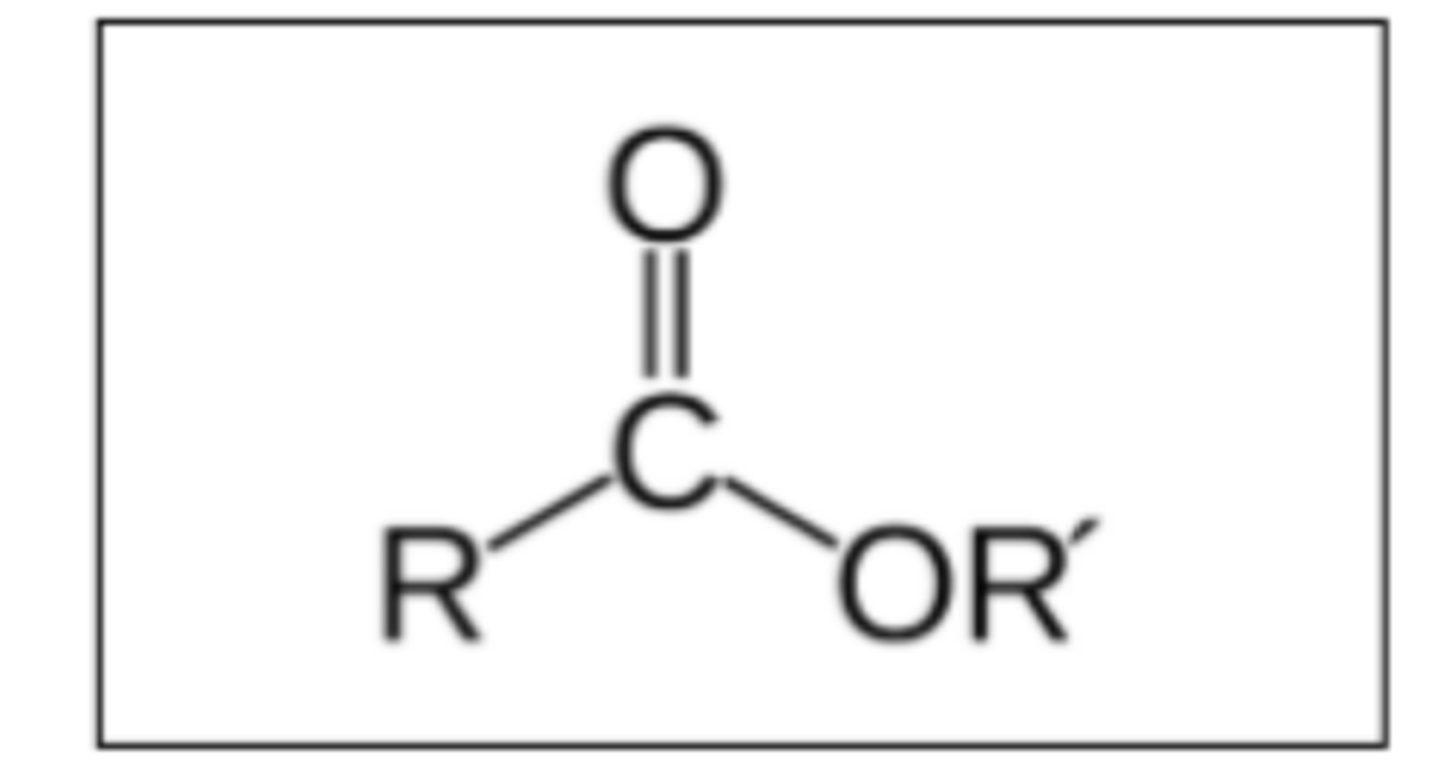
Draw and label the structure of an ester functional group (2)
What is the functional group isomer of esters?
Carboxylic acid
How is the name of an ester derived from its alcohol and carboxylic acid? (2)
Alcohol part (e.g. ethanol -> ethyl) + Acid part (e.g. ethanoic acid → ethanoate)
What is the ester formed from ethanol and propanoic acid? (1)
Ethyl propanoate
What is the ester formed from propan-1-ol and ethanoic acid? (1)
Propyl ethanoate
What happens when esters are hydrolysed using water and a dilute acid catalyst? (1)
Esters are broken down into their carboxylic acid and alcohol components
What catalyst is used for the hydrolysis of esters with water? (2)
Dilute H₂SO₄ or HCl
Why does hydrolysis of esters using water result in a low yield of carboxylic acid and alcohol? (1)
The reaction is reversible, so only partial hydrolysis occurs
What is the first step in the alternative 2-step method for hydrolysing esters with alkali? (1)
Add NaOH to hydrolyse the ester into an alcohol and a carboxylate salt
What happens during the second step of the alternative 2-step method for ester hydrolysis? (2)
- An acid such as H₂SO₄ or HCl is added
- To convert the carboxylate salt into a carboxylic acid
Write the reaction for the hydrolysis of methyl ethanoate with NaOH. (2)
- CH₃COOCH₃ + NaOH → CH₃COO⁻Na⁺ + CH₃OH
- Sodium ethanoate and methanol
What are the products of hydrolysing an ester with alkali followed by acid? (1)
A carboxylic acid and an alcohol
Why is an excess of dilute acid required in the second step of the 2-step ester hydrolysis? (1)
To fully convert the carboxylate salt back into the carboxylic acid
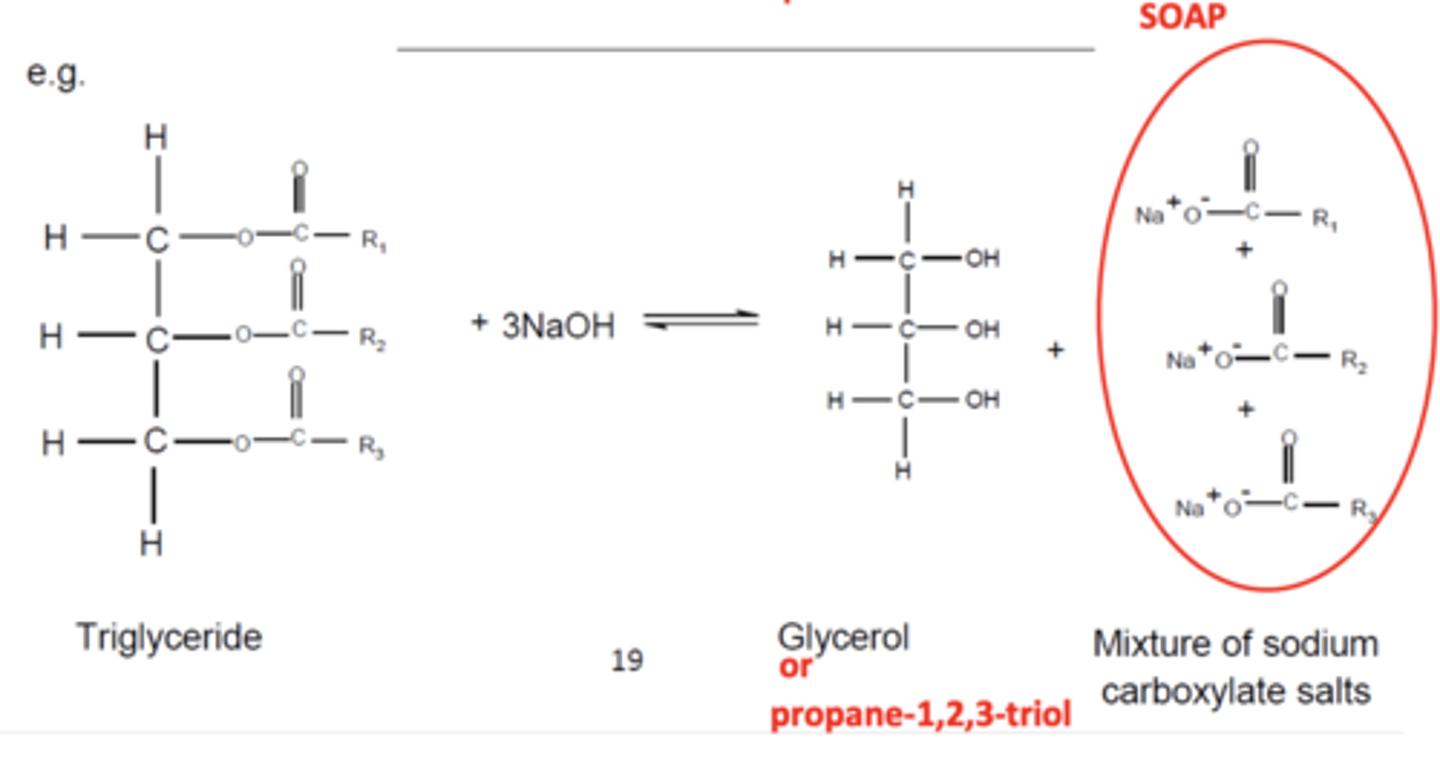
What are triglycerides? (1)
Triglycerides are triesters of long-chain carboxylic acids (fatty acids) and propane-1,2,3-triol (glycerol)
How can triglycerides be hydrolysed? (2)
- Using a hot NaOH solution
- To form glycerol and sodium salts of long-chain carboxylic acids
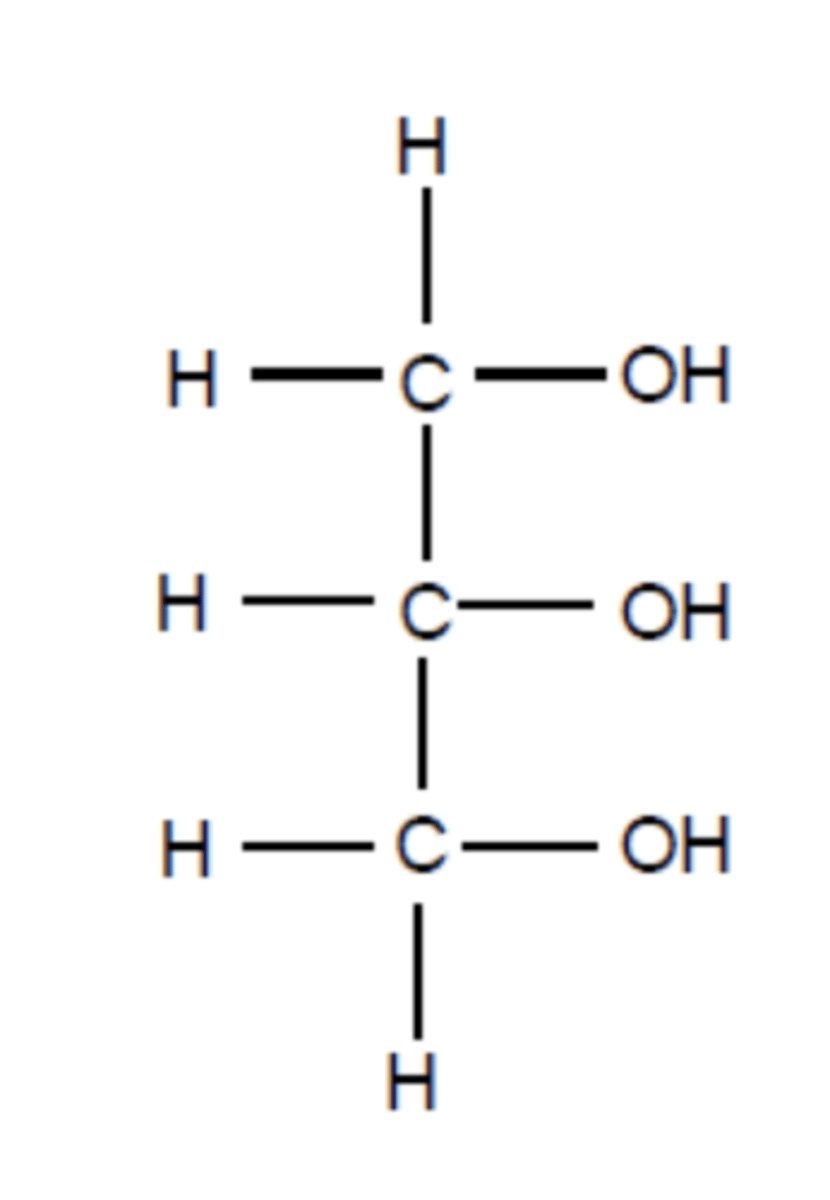
What is the chemical structure of glycerol? (1)
Propane-1,2,3-triol
Why are sodium carboxylate salts important? (1)
Sodium carboxylate salts are used in the manufacture of soaps
Draw the equation for the reaction of the formation of soap (4)
Why does glycerol dissolve easily in water? (2)
1. Glycerol has three -OH bonds
2. Which form hydrogen bonds easily with water
What are the uses of glycerol? (4)
1. Cosmetics industry
2. Food
3. Glues (to prevent materials from drying too quickly)
4. Wine
What is biodiesel? (2)
- Biodiesel is a liquid fuel consisting of a mixture of methyl esters of long-chain carboxylic acids
- Derived from vegetable oils
What is a biofuel? (1)
A biofuel is a fuel produced from renewable biological sources
How is biodiesel produced from vegetable oils? (3)
- Made by reacting vegetable oils, such as rapeseed oil
- With methanol (CH₃OH) in the presence of a strong acid or alkali catalyst
- To form a mixture of methyl esters
Why is biodiesel considered renewable? (1)
Biodiesel is renewable because it is made from oils derived from crops such as rapeseed
Draw an example reaction for the formation of biodiesel (5)
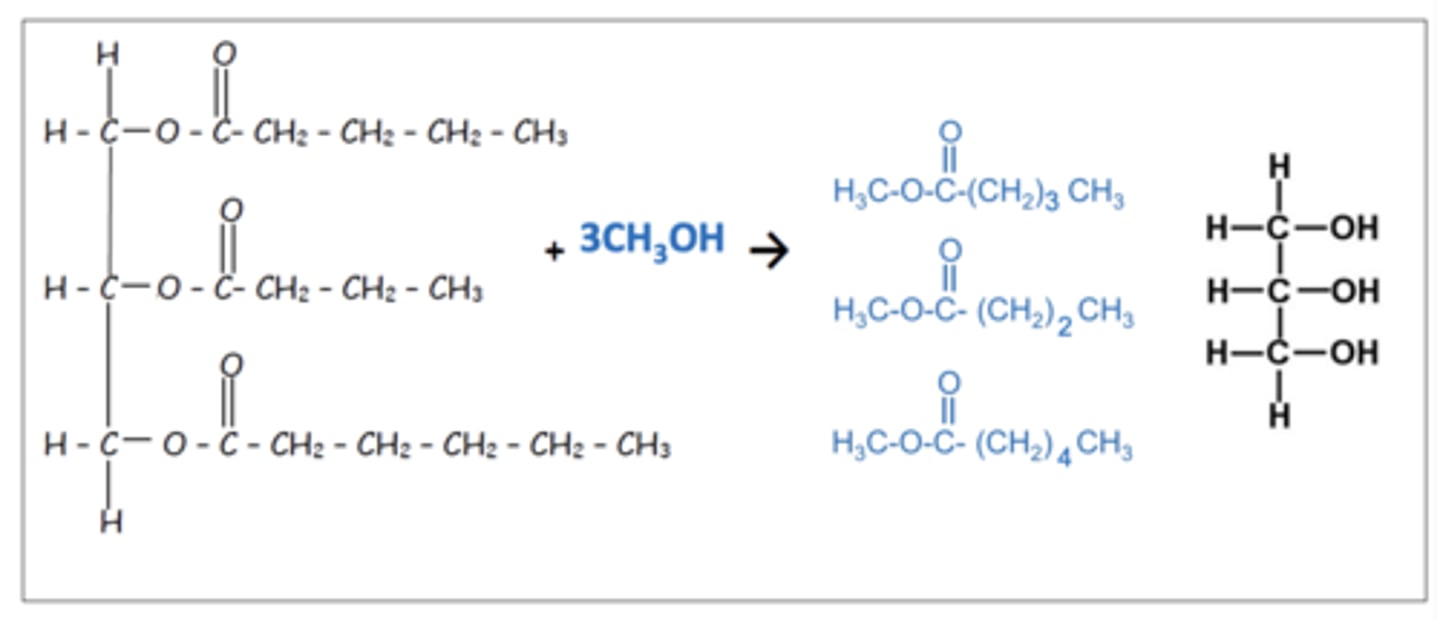
What are the raw materials used to make bioethanol? (1)
Sugar cane and sugar beet
What are the raw materials used to make biodiesel? (1)
Vegetable oil
How is bioethanol made? (1)
By fermentation
How is biodiesel made? (1)
By reacting vegetable oil with an alcohol and HCl
Where is bioethanol commonly used? (2)
- In cars as a mixture with petrol
- Widely used in Brazil
Where is biodiesel commonly used? (3)
In cars, buses, and vans as a mixture with diesel
How widely available is bioethanol in the UK? (1)
It is increasingly available
How widely available is biodiesel? (1)
It can be home-made and is available from some petrol stations
What two functional groups can carboxylic acids be turned into for use in organic synthesis? (1)
Acyl chlorides and acid anhydrides
How can carboxylic acids be turned into acyl chlorides? (1)
By replacing the -OH group of the carboxylic acid with a chlorine atom
Why is the carbon atom in an acyl chloride susceptible to nucleophilic attack? (2)
1. Chlorine and oxygen are more electronegative than carbon
2. Making the carbon atom δ⁺
What is the name of CH₃COCl? (1)
Ethanoyl chloride
What is the name of CH₃CH₂COCl? (1)
Propanoyl chloride
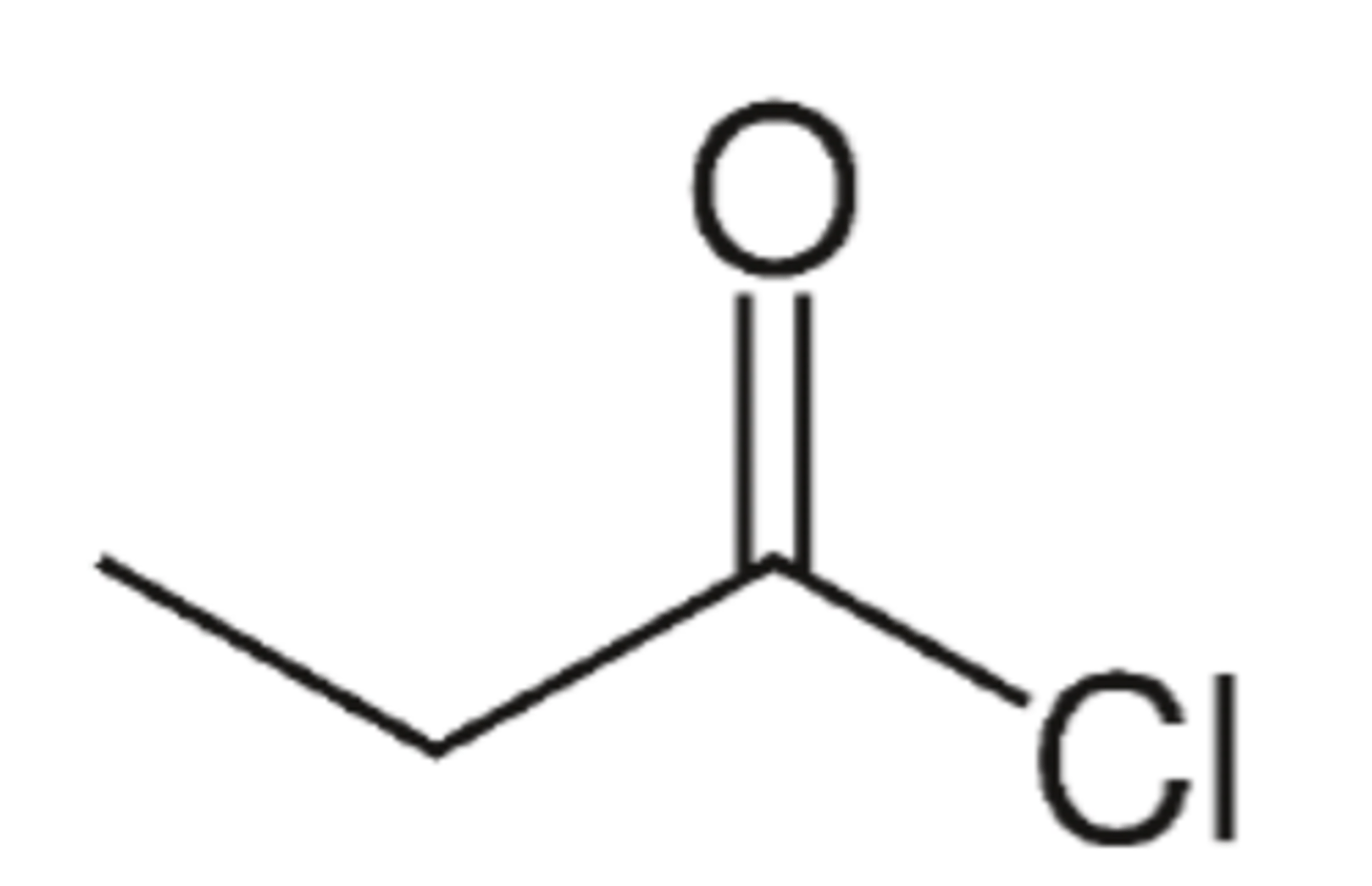
Draw the skeletal formula for propanoyl chloride (3)
What happens to the -OH group in carboxylic acids to form acid anhydrides? (1)
The -OH group of the carboxylic acid is replaced by -OCOR
How are acid anhydrides formed? (1)
When two molecules of carboxylic acids join together with the elimination of water
What is the general formula of an acid anhydride? (1)
(RCO)₂O
What is the name of the acid anhydride formed by the elimination of water from two molecules of ethanoic acid? (1)
Ethanoic anhydride
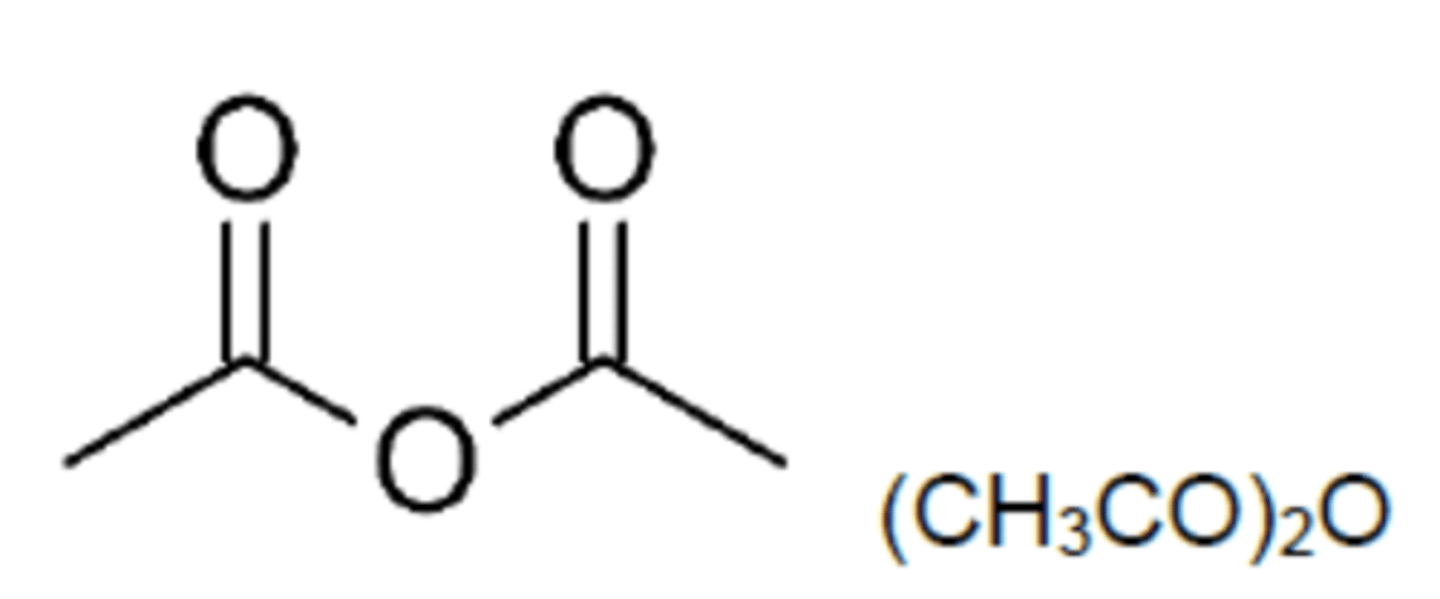
Draw the structure of ethanoic anhydride (3)
What is a good leaving group in carboxylic acid derivatives? (1)
A stable species that is removed during a chemical reaction
What are the leaving groups in acyl chlorides and acid anhydrides? (1)
- Acyl chloride: Cl⁻
- Acid anhydride: -OCOR
What by-product is formed when acyl chlorides react? (1)
HCl
What by-product is formed when acid anhydrides react? (1)
A carboxylic acid
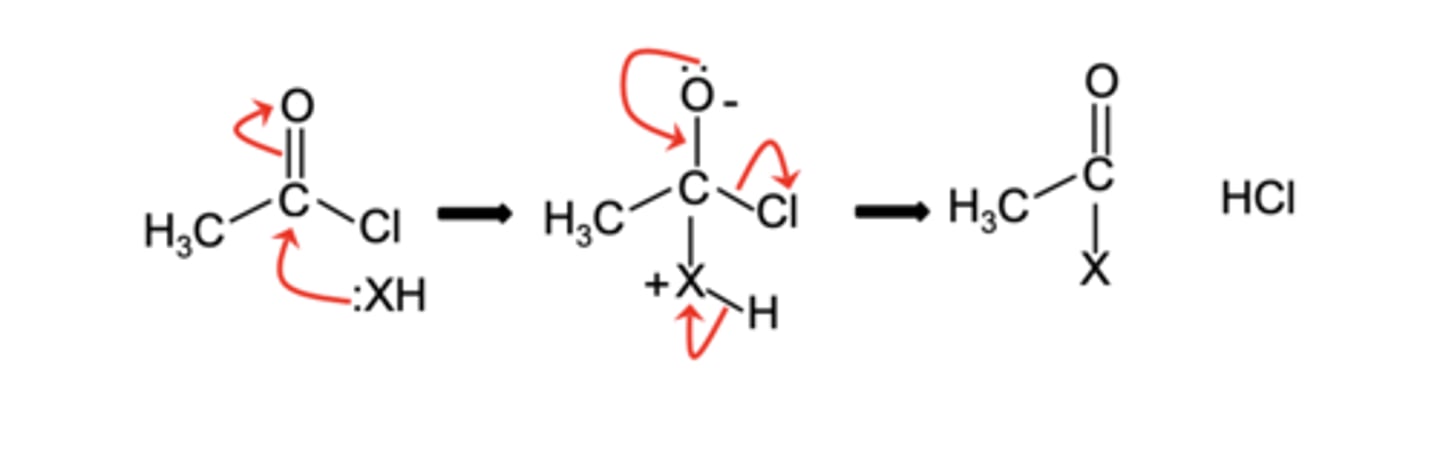
Draw the general mechanism for the reaction of acyl chlorides with nucleophiles in a nucleophilic addition-elimination reaction (4)
How are carboxylic acids formed from primary alcohols? (1)
Primary alcohols are oxidised by acidified potassium dichromate to form carboxylic acids.
Write the reaction for the oxidation of a primary alcohol to a carboxylic acid. (2)
RCH₂OH + 2[O] → RCOOH + H₂O
How are carboxylic acids formed from aldehydes? (1)
Aldehydes are oxidized by acidified potassium dichromate to form carboxylic acids
Write the reaction for the oxidation of an aldehyde to a carboxylic acid. (1)
RCHO + [O] → RCOOH
How do acyl chlorides react with water? (2)
Acyl chlorides react with water to form carboxylic acids and HCl
Write the reaction for the hydrolysis of an acyl chloride. (2)
RCOCl + H₂O → RCOOH + HCl
Give an example of an acyl chloride hydrolysis reaction. (2)
CH₃COCl + H₂O → CH₃COOH + HCl
Draw the mechanism for the reaction of acyl chlorides with water in a nucleophilic addition-elimination reaction to form water (4)
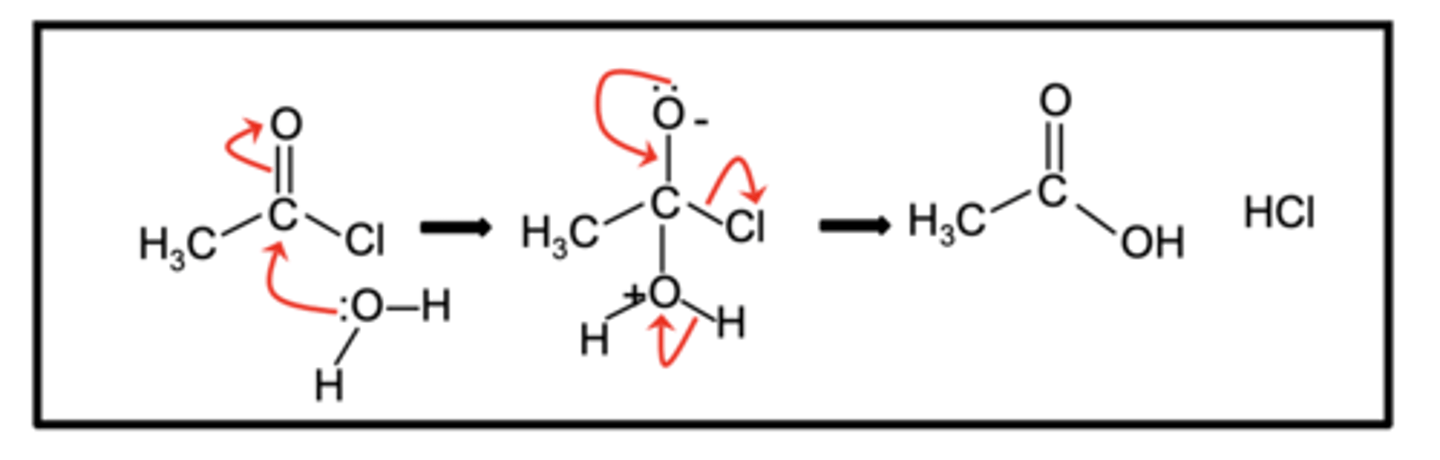
What do acid anhydrides form when they react with water? (2)
Two molecules of carboxylic acids
Write the general reaction for the hydrolysis of an acid anhydride. (2)
(RCO)₂O + H₂O → RCOOH + RCOOH
Provide an example of an acid anhydride reacting with water. (2)
(CH₃CO)₂O + H₂O → CH₃COOH + CH₃COOH
How do acyl chlorides react with alcohols to form esters? (2)
Acyl chlorides react with alcohols via nucleophilic addition-elimination to form esters and HCl
Write the general equation for the reaction of acyl chlorides with alcohols. (2)
RCOCl + CH₃OH → RCOOCH₃ + HCl
Provide an example of an acyl chloride reacting with an alcohol. (2)
CH₃COCl + CH₃OH → CH₃COOCH₃ + HCl
Draw the mechanism for the reaction of acyl chlorides with alcohols in a nucleophilic addition-elimination reaction to form esters (4)
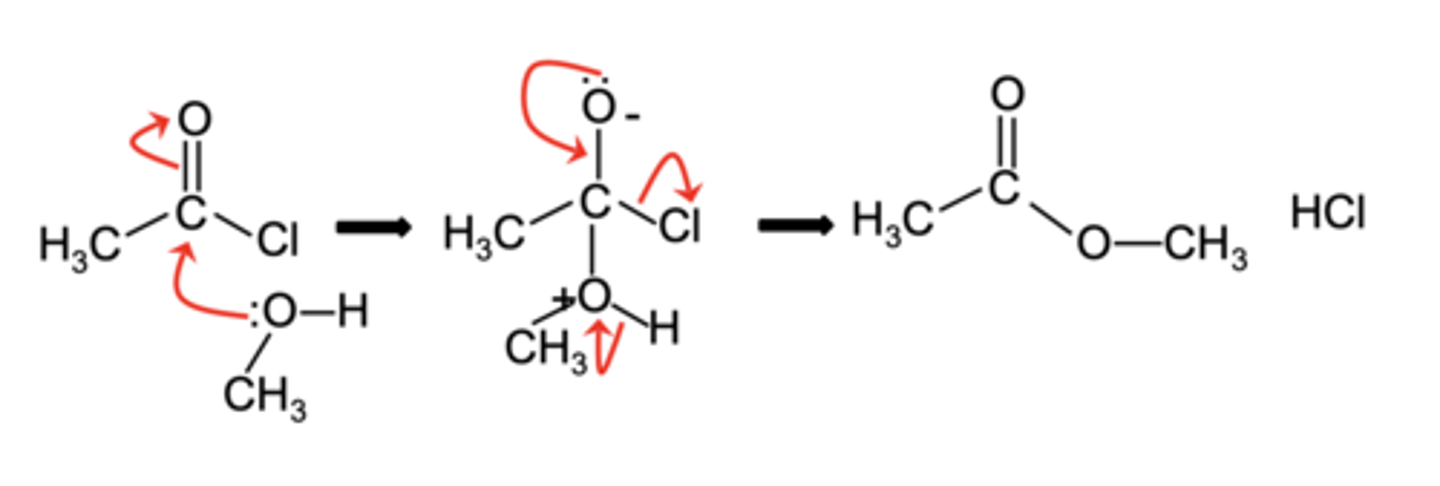
Why are acyl chlorides preferred to carboxylic acids for ester formation? (3)
- No acid catalyst is required (Esterification uses H₂SO₄).
- A purer product is obtained.
- The yield of ester is higher as the reaction is not reversible
What is the reaction equation for acid anhydrides with alcohols to form esters? (1)
(RCO)₂O + CH₃CH₂OH → RCOOCH₂CH₃ + RCOOH
Provide an example of an acid anhydride reaction with an alcohol to form an ester. (1)
(CH₃CO)₂O + CH₃CH₂OH → CH₃COOCH₂CH₃ + CH₃COOH
Why are acid anhydrides preferred to acyl chlorides for ester formation? (4)
1. Acid anhydrides are cheaper.
2. Acid anhydrides react less exothermically, making the reaction less violent/dangerous.
3. Acid anhydrides are less vulnerable to hydrolysis.
4. No corrosive HCl is formed.
What is ethanoic anhydride's advantage over ethanoyl chloride in synthesizing aspirin? (3) RP10
- Ethanoic anhydride is cheaper.
- Less corrosive and less vulnerable to hydrolysis.
- Less dangerous to use.
What is the main use of ethanoic anhydride in aspirin synthesis? (1) RP10
It is used in the manufacture of 2-ethanoyloxybenzenecarboxylic acid (aspirin)
What functional groups are involved in the synthesis of aspirin? (2) RP10
- Alcohol group in salicylic acid.
- Ester group in aspirin
Outline the first four steps of the method for synthesising aspirin. (4) RP10
1. Weigh out the given mass of salicylic acid.
2. Transfer the contents into a pear-shaped flask using a glass rod.
3. In the fume cupboard, add ethanoic anhydride and phosphoric acid.
4. Add anti-bumping granules to prevent violent boiling
What is the role of the water-ice bath during aspirin synthesis? (1) RP10
To allow complete crystallisation of aspirin
How is the purified aspirin obtained after crystallisation? (2) RP10
1. Filter the sample using a Buchner funnel under reduced pressure.
2. Wash the sample thoroughly with deionized water to remove impurities
What is the final step in drying the aspirin after filtration? (1)
Pat the sample dry using filter paper and place it in a fume hood for further drying
Draw and label the apparatus for a Buchner funnel under reduced pressure (5) RP10
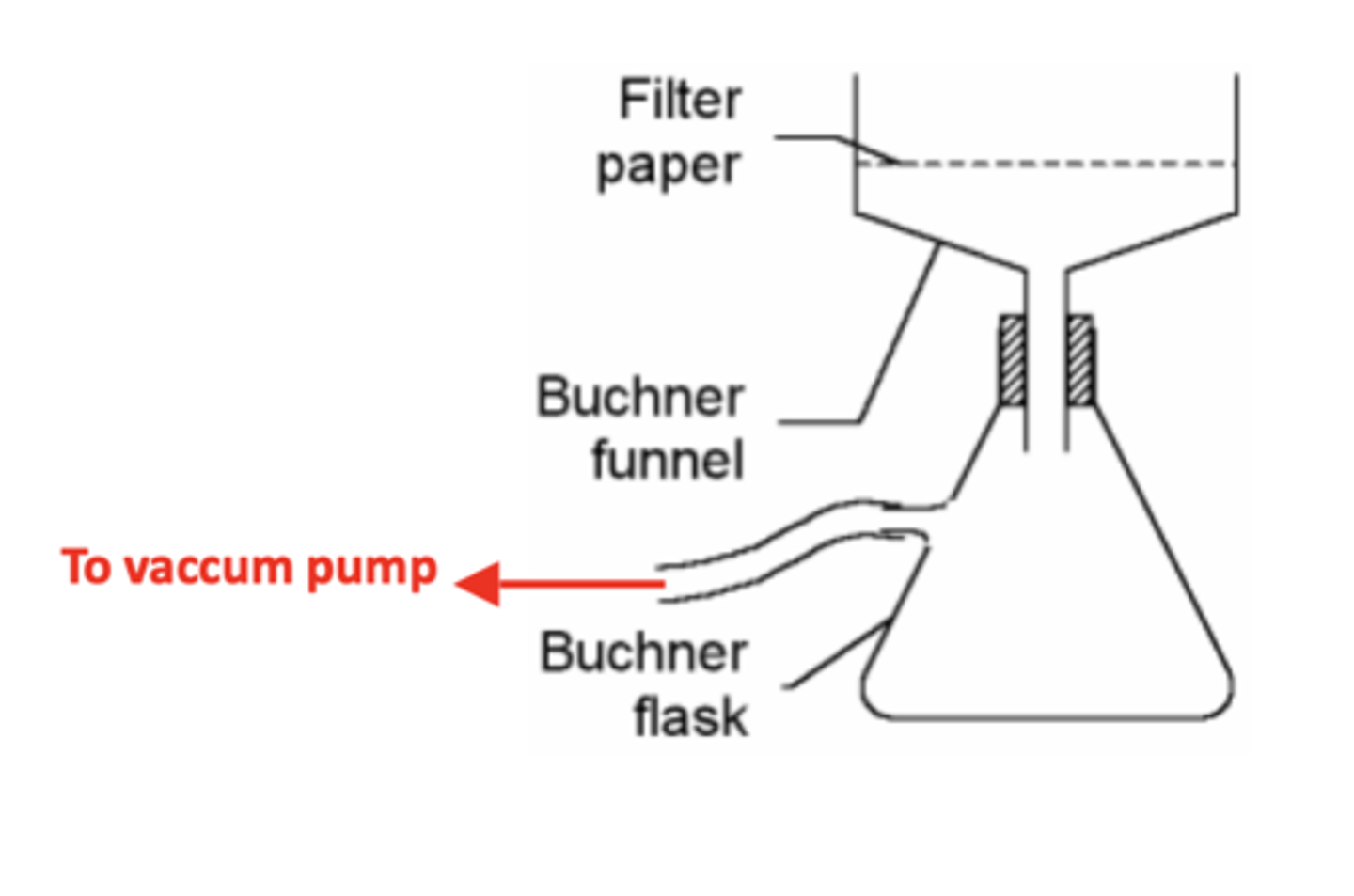
What is the suffix for naming amides? (1)
-anamide
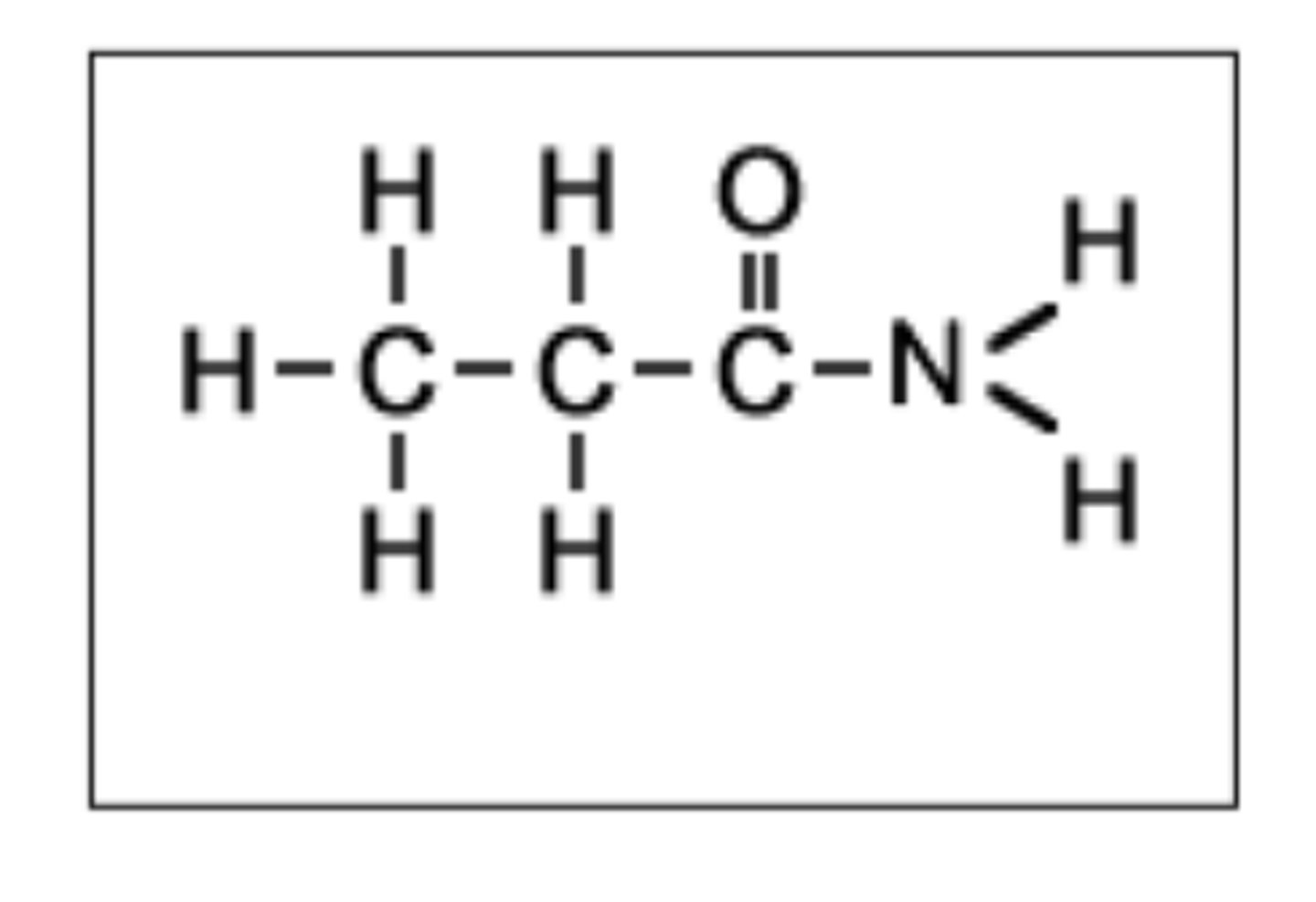
Draw the structure of propanamide (3)