O. Chem COM Exam 1
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Ionic bonding
Occur between metals, losing electrons, and nonmetals, gaining electrons
Covalent bonding
The interatomic linkage that results from the sharing of an electron pair between two atoms
Valence Shell Electron Pair Repulsion Theory (VSEPR)
Electron pairs (both shared and unshared) in the outermost energy level try to get as far apart as possible; determines shape of molecule
Linear
Central no unshared pairs; 2 or 3 atoms
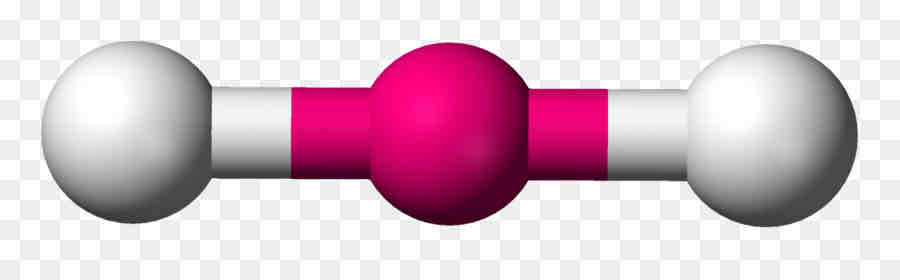
Linear Angle
180
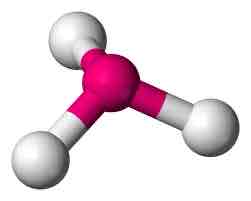
Bent
Central 1 or 2 unshared pairs; 3 atoms
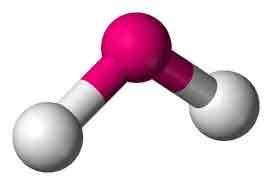
Bent angle
104.5
Trigonal planar
Central no unshared pairs; 4 atoms
Trigonal planar Angle
120
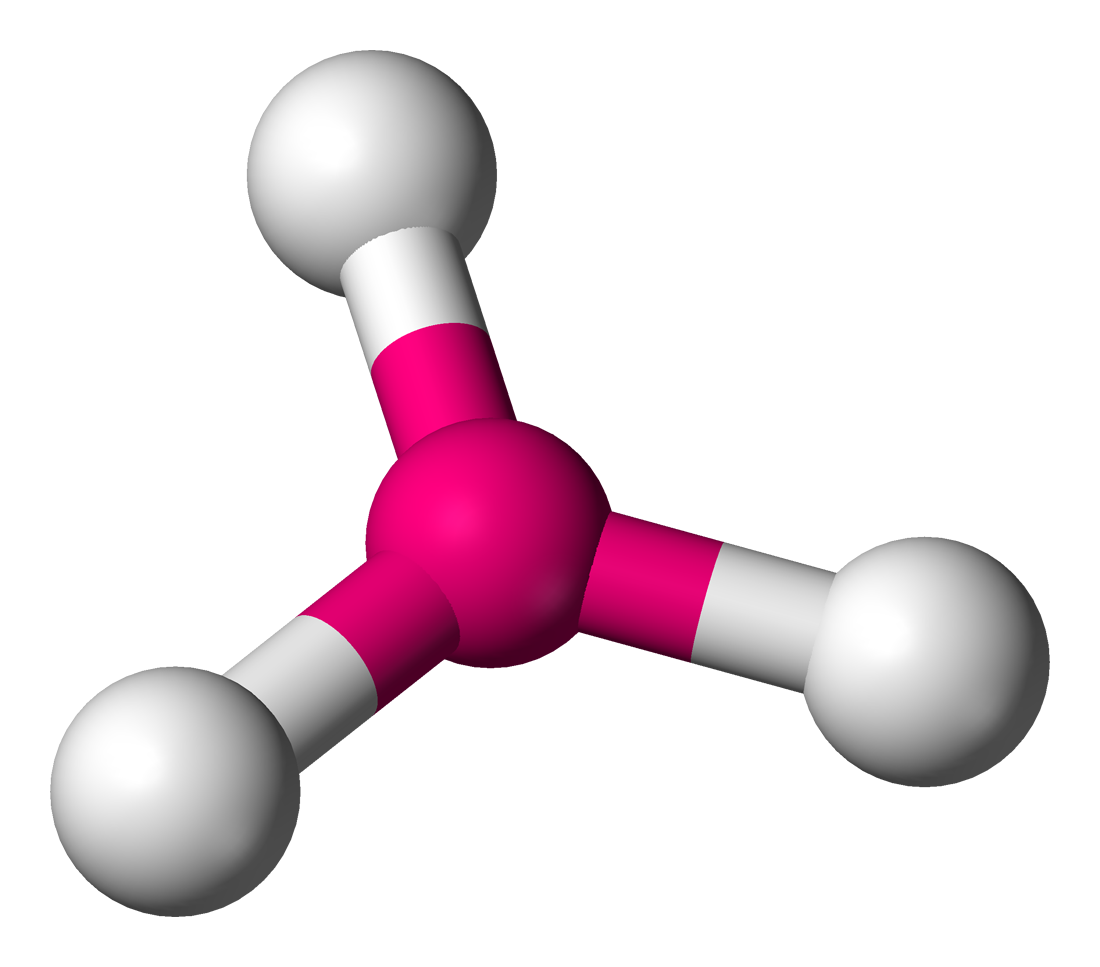
Trigonal Pyamid
Central 1 unshared pair; 4 atoms
Trigonal pyramid angle
107.5
Tetrahedral
No unshared; 5 atoms
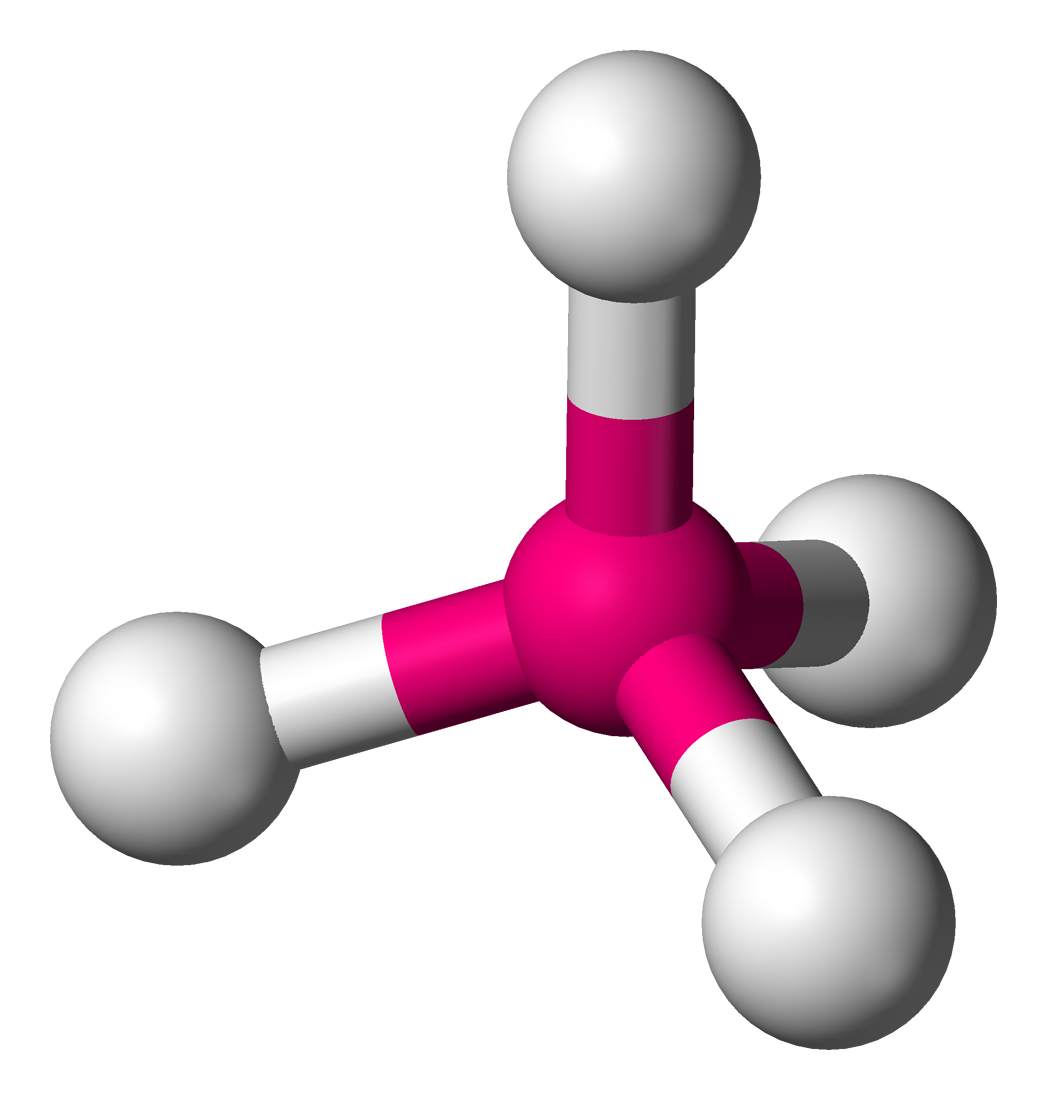
Tetrahedral angle
109.5
Polar
A covalent bond between two atoms where the electrons forming the bond are unequally distributed
Non-polar
A covalent bond between two atoms where the electrons forming the bond are equally distributed
Period
Horizontal rows periodic table
Group
Vertical columns periodic table