Organic Chemistry I: Chapter 9 & 10 Mechanism Alkynes
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
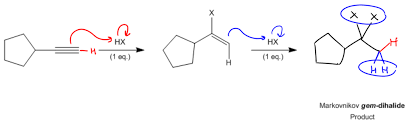
Hydrohalogenation
Reagent(s): HX or Excess HX
Adds What: X or X and X
Regiochemistry: Mark (unless ROOR then anti Mark)
Steriochemistry: N/A
Elimination of Alkyl dihalide
alkyl halide goes through two eliminations by a strong base
E2 reactions
terminal alkyne will be de-protonated —→ quench step is required
HBR will make one halide added and excess will result in two halides added to the carbon
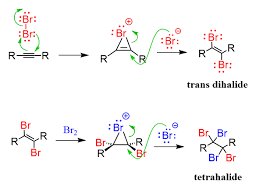
Halogenation
Reagent(s): X2 (results in alkene with two added X groups) or XS X2 (results in alkane with four added Xs)
Adds What: 2 X groups added anti from each other on alkene or 4X added to alkane to make tetrahalide
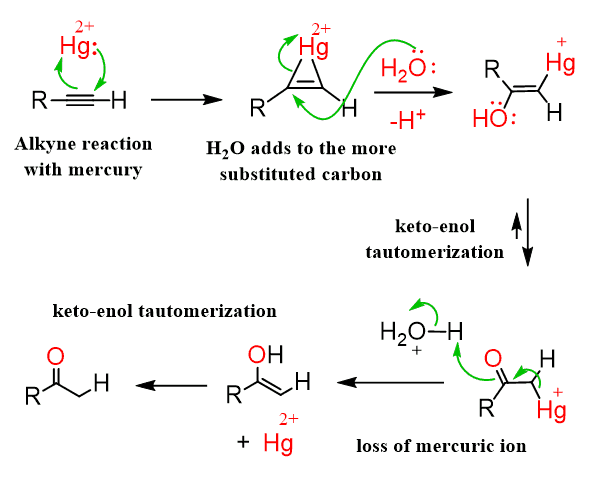
Hydration
Reagents: H3 O+/HgSO4 —→ enol —→ taumerization for Ketone
Extra considerations for alkynes:
o H3 O+ alone won’t work – HgSO4 is necessary
o Hydration of an alkyne gives an enol. New mechanism: tautomerization (acidic
conditions).
o No oxymercuration-demercuration (no vinyl carbocation formation, so it’s not
necessary)
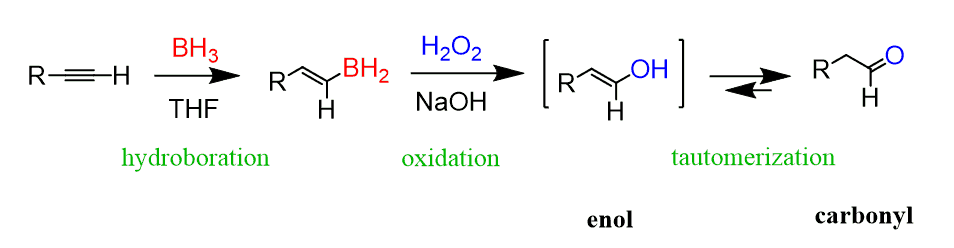
Hydroboration-oxidation
Reagents: 1. BH3 2. H2O2, NaOH —→ enol —→ taumerization for aldehyde
Extra considerations for alkynes:
o Hydration of an alkyne gives an enol. New mechanism: tautomerization (basic
conditions).
o BH3 generally gives multiple hydride additions, so we use a bulky dialkylborane (HBR2 )
such as 9-BBN or dicyclohexylborane (Cy2 BH).
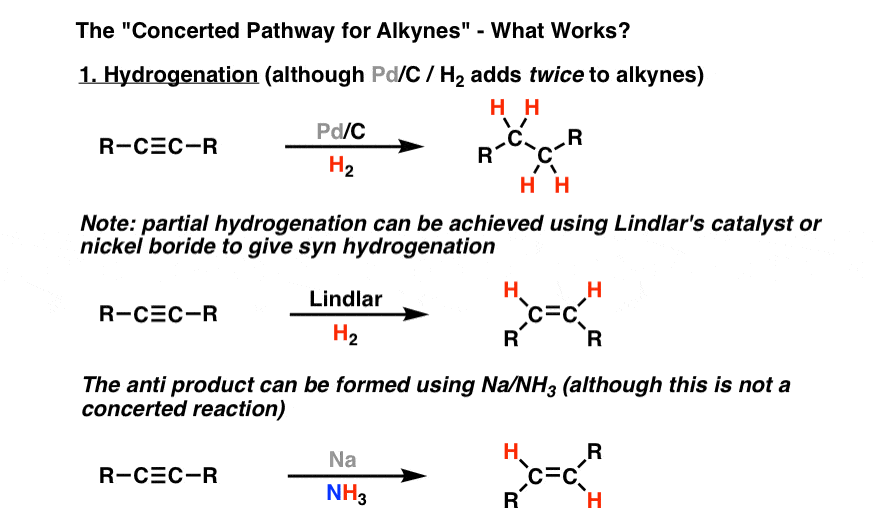
Hydrogenation
Reagents: metal, Cold Products: alkane
Reagents: Na/NH3 Products: trans alkene
Reagents: H2 / Lindar Products: cis alkene
Extra consideration for alkynes: single reduction to give cis or trans alkene selectively
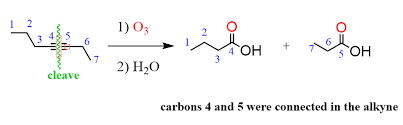
Ozonolysis
Reagents: O3/H2O Products: Ketones with O and R group
Extra considerations for alkynes:
o When the triple bond of an alkyne is fully oxidized, followed by hydrolysis, carboxylic
acids are produced.
o When a terminal alkyne undergoes ozonolysis, carbon dioxide is formed.
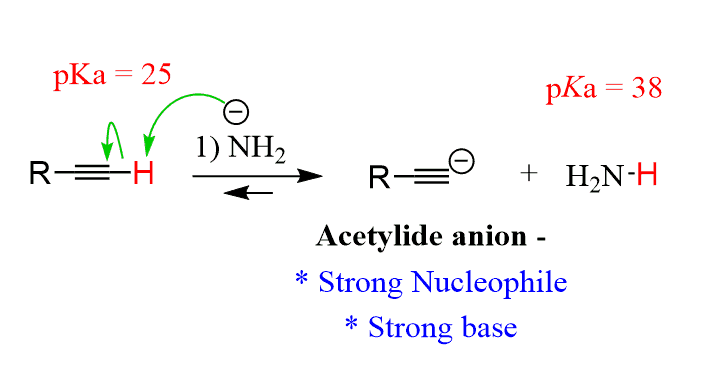
Deprotonation
Reagents:
Extra consideration for alkynes:
o Use a strong enough base (pKa of conjugate acid must be greater than 25).
o This SN2 reaction gives us our FIRST C-C BOND-FORMING REACTION!!!!!
o Alkynyl anions are strong bases and strong nucleophiles – this only works well with
primary and methyl alkyl halides.