Atomic Structure test
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
What term is defined as the region in an atom where an electron is most likely to be located?
Orbital
Which one of the following phases describes an Al atom?
A positively charged nucleus, surrounded by negatively charged electrons
The part of an atom that has an overall positive charge is called
The nucleus
Atoms are neutral because the number of
protons equals the number of electrons
Which conclusion directly resulted from the “gold foil experiment”?
Atoms are mostly empty space
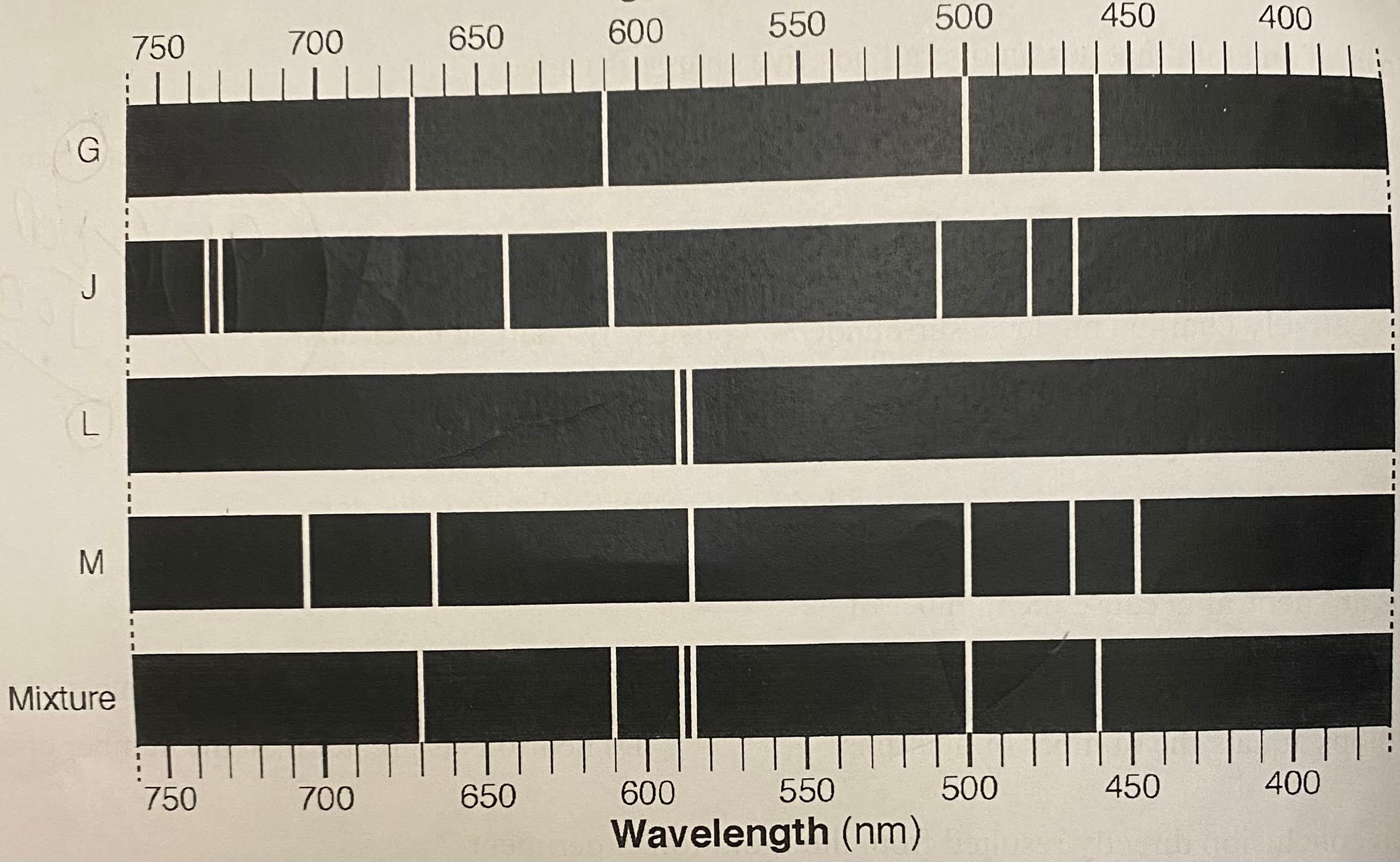
The bright-line spectra of four elements, G, J, L, and M, and a mixture of at least two of these elements is given in the table.
Which elements are present in the mixture?
G and L
What is the total number of neutrons in an atom of K-42?
23
The mass of a proton is approximately equal to the mass of
A neutron
Which one of the following particles has the least mass?
An electron
Which subatomic particles each have a mass of approximately 1 u?
Proton and Neutron
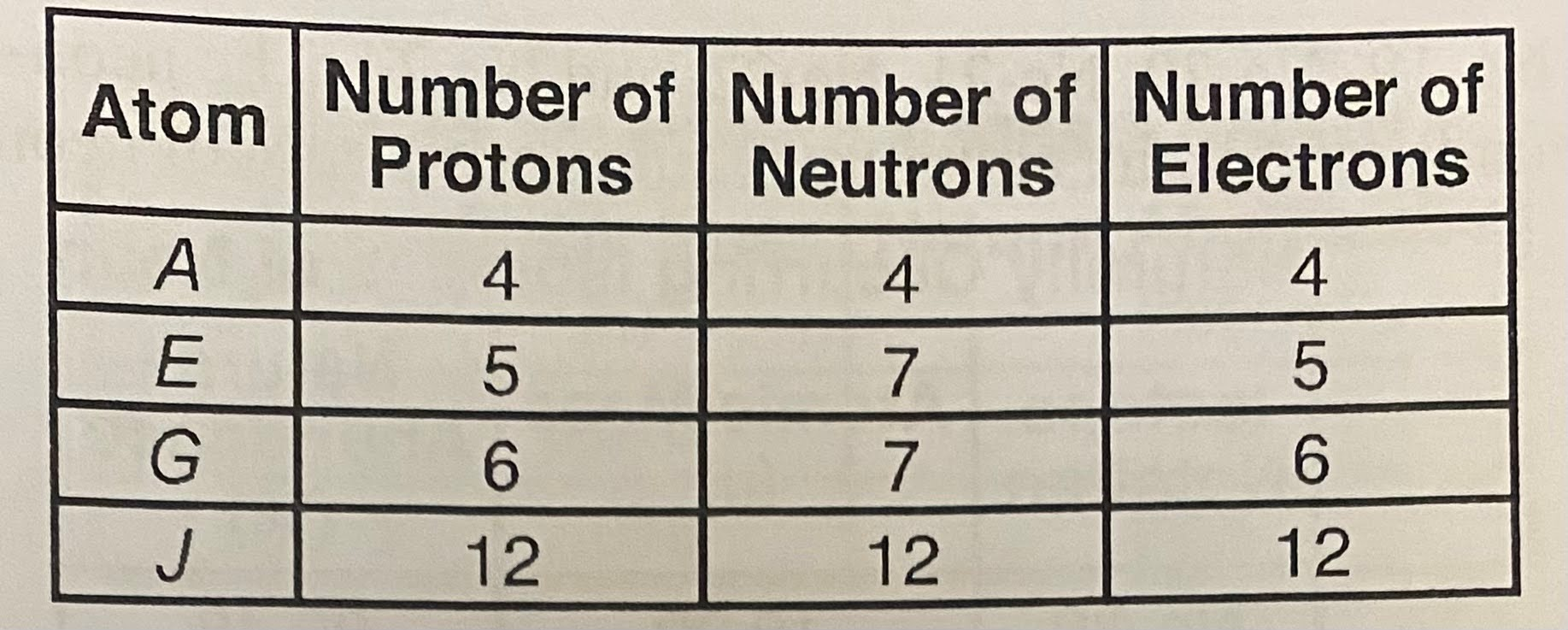
Given the table representing the subatomic particles in four different atoms,
Which atom has a mass of 12 u?
E
An atom that contains six protons, six neutrons, and six electrons has a mass of approximately
12 u
Compared to an atom of C-12, an atom of C-14 has a greater
mass number
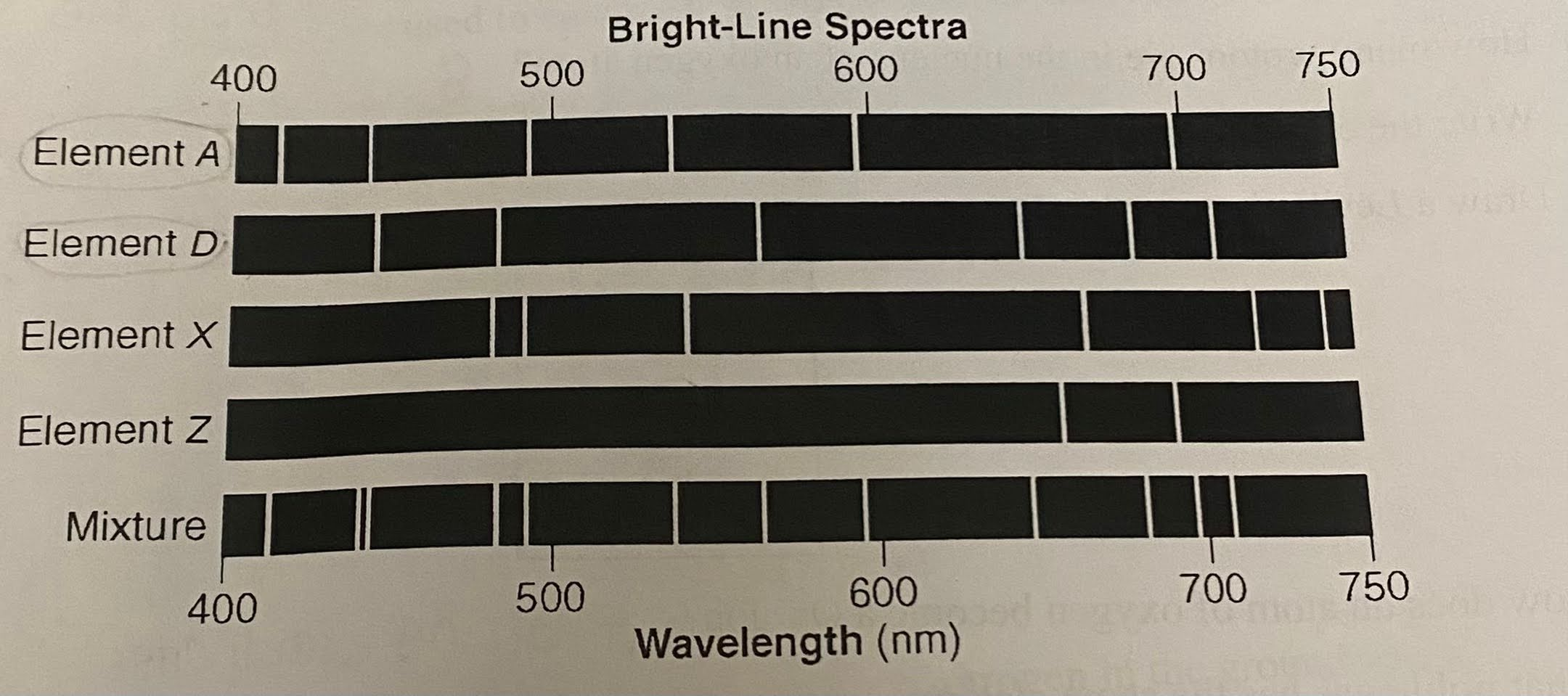
Write the letter of each element present in the mixture represented in the given diagram.
A, D
Tritium, hydrogen-3, is a radioisotope.
State the number of neutrons in an atom of tritium.
2 neutrons
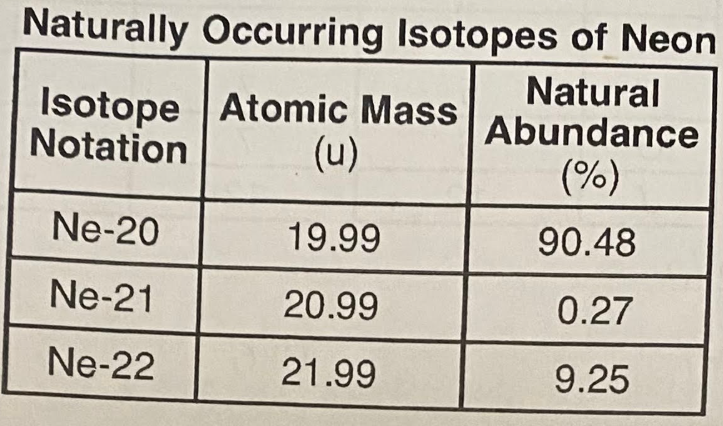
Some isotopes of neon are Ne-19, Ne-21, Ne-22, and Ne-24. The neon-24 decays by beta emission. The atomic mass and natural abundance for the naturally occurring isotopes of neon are shown in the table.
Show a numerical setup for calculating the atomic mass of neon.
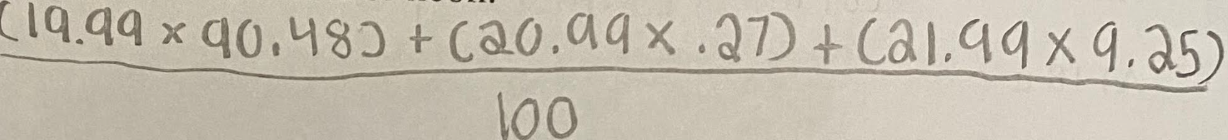
The element technetium, Tc, has several isotopes. The bright-line spectrum of technetium has been observed in the spectra of some stars.
State, in terms of protons and neutrons, why the various nuclides of technetium are isotopes of each other.
They have the same number of protons but a different number of neutrons.
How many protons are in the nucleus of an oxygen atom?
8
Write the electron configuration for an atom of oxygen in the ground state.
2 - 6
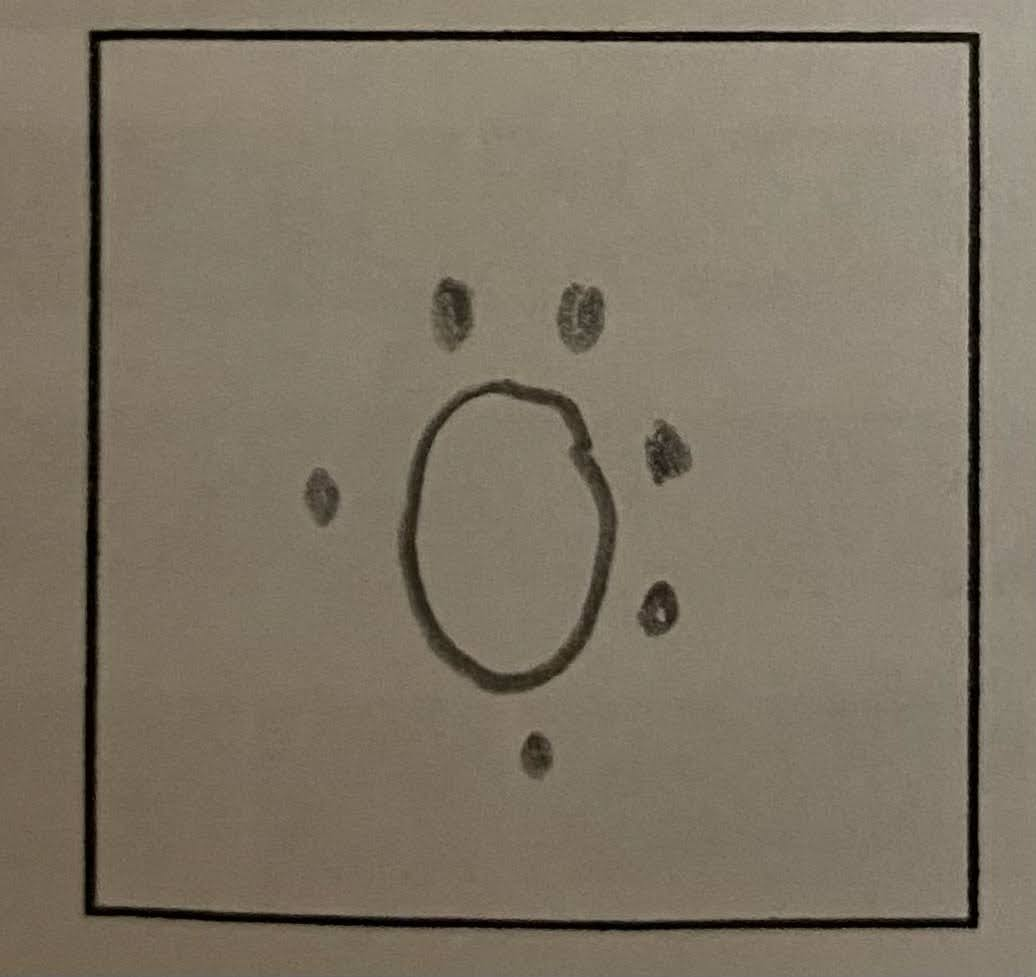
Draw a Lewis electron-dot diagram for an atom of oxygen.
How does an atom of oxygen become a O2- ion?
It gains electrons
What noble gas has the same electron configuration as O2-?
Both O2- and Ne have an electron configuration of 2- 8
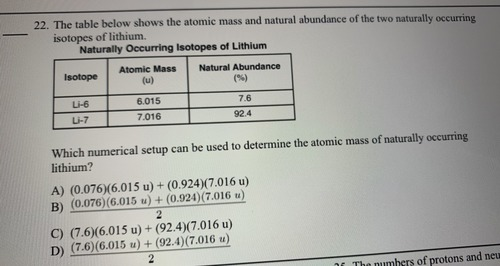
The table shows the atomic mass and natural abundance of the two naturally occurring isotopes of lithium.
What numerical setup can be used to calculate the atomic mass of silver?
D
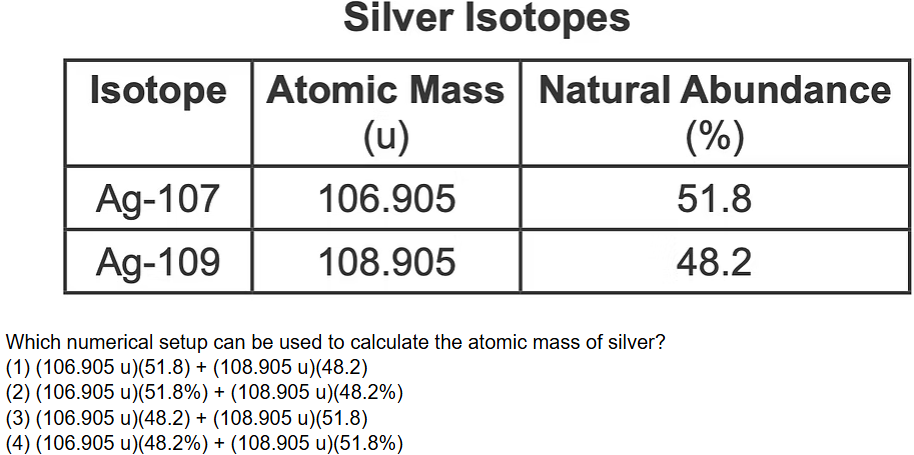
The atomic masses and natural abundances of the two naturally occurring isotopes of silver are shown in the table.
What numerical setup can be used to calculate the atomic mass of silver?
(106.905 u)(51.8%) + (108.905 u)(48.2%)
In all atoms of bismuth, the number of electrons must equal the
number of protons

Which Lewis electron-dot diagram represents an atom of nitrogen in the ground state?
D
Which electrons in a calcium atom in the ground state have the greatest effect on the chemical properties of calcium?
the two electrons in the fourth shell
An excited potassium atom emits a specific amount of energy when one of its electrons moves from
the fourth shell to the second shell
Elements that have atoms with stable valence electron configurations in the ground state are found in
Group 18
Which electron configuration represents the electrons in an atom of sodium in the ground state at STP?
2 - 8 - 1
which one of the following electron configurations represents the electrons in an atom of calcium in an excited state?
2 - 7 - 8 - 3
Which one of the following electron configurations represents the electrons of an atom in an excited state?
2 - 7- 3
Experimental evidence indicates that the nucleus of an atom
contains most of the mass of the atom
Which of the following statements is a part of Dalton’s atomic theory?
Different atoms combine in simple whole-number ratios to form compounds.