Topic 1 Principles of Chemistry PMT
1/108
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
109 Terms
solid ⇄ gas
Sublimation (s to g)
Deposition (g to s)
What is diffusion?
It is the net movement of particles from an area of high concentration to an area of low concentration.
What is a solubility curve?
It is a curve that shows how the solubility of a substance (in grams per 100 g of water) changes with temperature.
Outline the main assumptions of the kinetic theory of matter.
a) Matter is made up of atoms, molecules and ions of different sizes.
b) At the same temperature, small particles move faster than large particles
c) As temperature rises, the particles have more kinetic energy and move faster
d) Solids are made up of ordered arrangement of closely packed particles
e) Liquids do not have particles arranged regularly. Particles can move around.
f) In gases, the particles are far apart. They move fast. Their motion is random.
Explain what is meant by centrifuging
It is a method for separating out particles of different densities in a substance. It can be used to separate suspended solids (very small particles of solid) from the liquid they are suspended in. It is used when the particles are so small that they can’t be separated via filtration. In a centrifuge, the sample is spun at high rates. This forces the solid particles to settle down at the bottom of the tube. The liquid can be decanted.
What is an atom
An atom is the smallest particle of a chemical element that can exist.
What is an element
An element is substance made up of only one type of atom
How are the element listed and approximately how many are there?
They are listed in the periodic table; 118
Elements can be classified into two groups based on their properties; what are these groups?
Metals and Non-metals
Elements may combine through chemical reactions to form new products; what are these new substances called?
compounds
What is a compound
Two or more elements combined chemically in fixed proportions which can be represented by formulae
Do compounds have the same properties as their constituent elements?
No they have different Properties
What is a mixture? Does it have the same chemical properties as its constituent materials?
A mixture consists of two or more elements or compounds not chemically combined together; the constituent materials keep their own chemical properties, but the mixture may have different chemical properties (e.g. melting point) as a whole.
What are the methods through which mixtures can be separated (there are five)? Do these involve chemical reactions?
Filtration, evaporation/crystallisation, simple distillation, fractionaldistillation and chromatography; they do not involve chemical reactions
Describe and Explain simple distillation
Simple distillation is used to separate liquid from a solution — the liquid boils off and condenses in the condenser. The thermometer will read the boiling point of the pure liquid. Contrary to evaporation, we get to keep the liquid (it drips and is collected into a separate beaker).
Describe and explain crystallization/evaporation
Evaporation is a technique for separation of a solid dissolved in a solvent from asolvent (e.g. salt from H,0).I'he solution is heated until all the solvent evaporates; the solids stays in the vessel.Crystallisation is similar, but we only remove some of the solvent by evaporation toform a saturated solution (the one where no more solid can be dissolved). Then,we cool down the solution. As we do it, the solid starts to crystallise, as it becomes less soluble at lower temperatures. The crystals can be collected and separated from the solvent via filtration.
Describe and explain fractional distillation
Fractional distillation is a technique for separation of a mixture of liquids. It works when liquids have different boiling points.The apparatus is similar to the one of simple distillation apparatus, with the additional fractionating column placed on top of the heated flask.The fractionating column contains glass beads. It helps to separate the compounds. In industry, mixtures are repeatedly condensed and vapourised. The column is hot at the bottom and cold at the top. The liquids will condense at different heights of the column.
Describe and explain filtration
Filtration is used to separate an insoluble solid suspended in a liquid. Theinsoluble solid (called a residue) gets caught in the filter paper, becausethe particles are too big to fit through the holes in the paper.The filtrate is the substance (liquid) that comes through the filter paper.Apparatus: filter paper + funnel.
Describe and explain chromatography
Chromatography is used to separate a mixture of substances dissolved in a solvent. In paper chromatography, we place a piece of paper with a spot containing a mixture in a beaker with some solvent. The bottom of the paper has to be in contact with the solvent. The solvent level will slowly start to rise, thus separating the spot(mixture) into few spots (components).
Describe a paper chromatography experiment
a) A start line is drawn near the bottom of the paper. The mixture is spotted on the line.
b) A beaker is filled with small amount of solvent (it cannot touch or go above the start line when paper is placed in a beaker)
c) Paper is hung on a rod and placed in a beaker.
d) Solvent travels up the paper, thus separating the components.
e) Before solvent level reaches the end, the paper is taken out and the finish line ismarked. The paper is dried.
f) The procedure works when the components dissolve differently in the solvent. More soluble components travel further up the paper. Less soluble components have stronger attraction for the paper and travel less slowly with the solvent, therefore less further up the paper.
g) Paper is called the stationary phase - it doesn't move. Solvent is the mobile phase.
How is Rf calculated?
Distance moved by the spot (solute component) / distance moved by solvent
In a paper chromatography experiment, a compound A was found to have an R, value of0.85 - what does it tell you about the compound?
It has a higher affinity for the solvent than for the paper.
What is a separating funnel?
A separatory funnel is an apparatus for separating immiscible liquids.Two immiscible liquids of different densities will form two distinct layers inthe separatory funnel.
We can run off the bottom layer (the liquid with greater density) to a separate vessel.
Describe the plum-pudding model
The atom is a ball of positive charge with negative electrons embedded in it.
Describe the Bohr/nuclear model and how it came about
The nuclear model suggests that electrons orbit the nucleus in energylevels (at specific distances from nucleus) — it came about from the alphascattering experiments conducted by Ernest Rutherford and two students
Later experiments led to the discovery of smaller, positive particles in the nucleus; what are these particles called?
protons
What did the work of James Chadwick provide evidence for?
The atom has a small central nucleus (made up of protons and neutrons)around which there are electrons.
Describe the structure of an atom
The atom has a small central nucleus (made up of protons and neutrons)around which there are electrons.
State the relative masses and relative charge of the proton, neutron and electron
Masses: 1, 1, very small (respectively)
Charges: 1, 0, -1 (respectively)
Explain why atoms are electrically neutral.
They have the same number of electrons and protons
What is the radius of a nucleus ?
0.1nm
What is the radius of a nucleus and what is it compared to that of the atom?
1x 10" m and less than 1/10000 of the radius of the atom.
What name is given to the number of protons in the nucleus?
Atomic number
Atoms of the same element have the same number of which particle in the nucleus?
Protons
Where is the majority of mass of an atom?
The nucleus
What is the mass number?
The total number of protons and neutrons in the nucleus.
How does one calculate the number of neutrons using mass number and atomic number?
| Subtract the atomic number from the mass number. |
What is an isotope? Do isotopes of a certain element have the same chemical properties?
Atoms of the same element (same proton number) that have a differentnumber of neutrons.They have the same chemical properties as they have the same electronic structure.
What is the relative atomic mass?
The average mass value of one atom (taking into account the abundance ofisotopes), compared to 1/12 of the mass of one carbon-12 atom.
Give the electronic configurations of He (2), Be (4), F (9), Na (11), and Ca(20) to demonstrate how shells are occupied by electrons.
He, Be, F, Na, Ca configurations (respectively):222272,812,8,8,2
Describe the properties of noble gases. Discuss the trends in properties down the group.
Non-metals, colourless gases at room temperature, low boiling points,unreactive (full outer shell; they don’t easily accept or lose electrons).The boiling point increases down the group, as the atoms get heavier
Miscible
Miscible refers to the substances (particularly liquids) that mix together in allproportions, e.g. water and alcohol. Water and oil are immiscible, i.e. they do notmix.
The relation between solute, solvent and solution
A solute is a substance that is dissolved in a solvent. Together they form a solution.
The columns of the periodic table are called?
Groups
The rows of the periodic table are called...?
Periods
Are elements in the same group similar or different?
They may have similar chemical properties, as they have the same number of outer shell electrons.
In terms of energy levels, what are thedifferences between elements of the sameperiod?
They have the same number of energy levels
Electrons occupy particular energy levels, with eachelectron in an atom at a particular energy level; whichavailable energy level do electrons occupy?
The lowest available energy level
The elements of Group 0 are more commonlyknown as...?
The noble gases
What makes the periodic table periodic?
Similar properties of elements occur at regular intervals |
Elements in the same group have the same number of electrons in their outer shell; what does this tell us about their chemical properties?
They have similar chemical properties
In terms of shells, what is the difference between elements in the same period?
They have the same number of shells
What change in shell number is seen as one moves down a group?
The number of shells increases
Early periodic tables were incomplete andelements were placed in inappropriate groups ifwhat was to be followed?
Strict order of atomic weights
Knowledge of what made it possible to explain why the order based on atomic weights was not always correct?
Isotopes
Early periodic tables were incomplete and elements were placed in inappropriate groups if what was to be followed?
Leaving gaps; atomic weights
The majority of elements are...?
Metals
Elements that react to form positive ions are...?
Metals
Elements that do not form positive ions are...?
Non-metals
Elements in Group 1 are known as...?
The alkali metals
Elements in Group 7 are known as...?
The halogens
What is ionic bonding
How are ionic compounds held together?
How are ionic compounds held together?
They are held together in a giant lattice. It's a regular structure that extends in all directions in a substance.Electrostatic attraction between positive and negative ions holds thestructure together.
State properties of ionic substances
High melting and boiling point (strong electrostatic forces between oppositely charged ions). Do not conduct electricity when solid (ions in fixe (ummm Conduct when molten or dissolved in water - iong
What is important when working out a formula of an ionic compound?
Ionic compounds are electrically neutral, i.e. positive and negative charges balance each other.
How are ionic compounds formed? Explain interms of MgO case.
Reaction of a metal with a non-metal.Electron transfer occurs - metal gives away its outer shell electrons tonon-metal.
Mg is in Group Il, so has 2 available outer shell electrons.
O is in Group VI, so can accept 2 electrons to get a full outer shell configuration.
terms of MgO case.Mg becomes Mg?* and O becomes O?~ (oxide).
What is a covalent bond?
Covalent bond is a shared pair of electrons between two atoms.
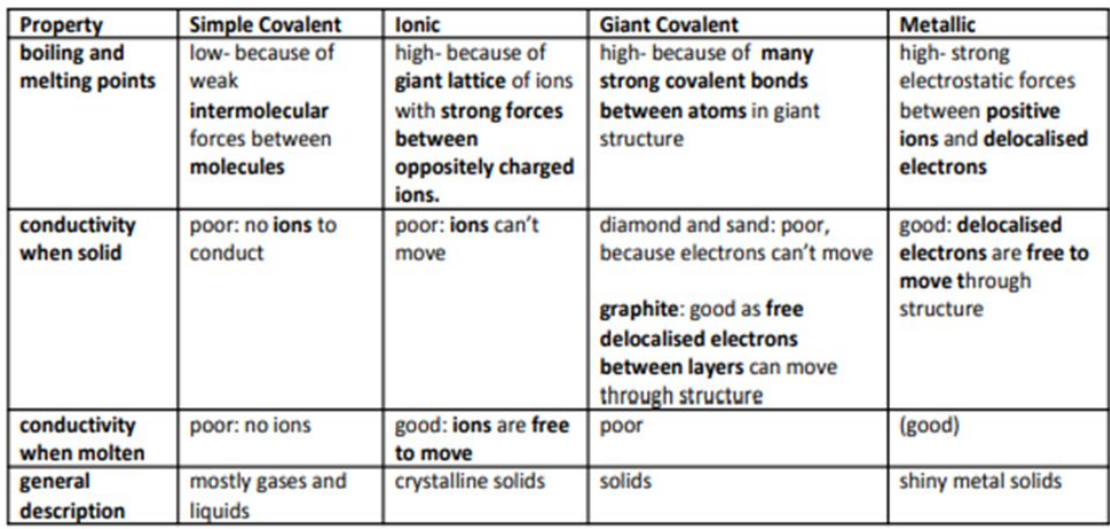
Describe the structure and properties of simple molecular covalent substances
- Do not conduct electricity (no ions)
- Small molecules
- Weak intermolecular forces, therefore:- Low melting and boiling points
How do intermolecular forces change as the mass/size of the molecule increases?
They increase. That causes melting/boiling points to increase as well (more energy needed to overcome these forces).
What are giant covalent substances?
Solids, atoms covalently bonded together in a giant lattice.High melting/boiling points — strong covalent bonds.Mostly don’t conduct electricity (no delocalised e-)
Give examples of giant covalent structures. (3)
eg: Diamond, graphite, silicon dioxide (silica), etc.
Properties of diamonds
— four, strong covalent bonds for each carbon atom
— very hard (Strong bonds)
— very high melting point (strong bonds)
— does not conduct electricity (no delocalised electrons)
Properties of graphite
— three covalent bonds for each carbon atom
— layers of hexagonal rings
— high melting point
— layers free to slide as weak intermolecular forces between layers; soft, can be used as a lubricant
— conduct thermal and electricity due to one delocalised electron per each carbon atom
Properties of fulerene
— hollow shaped molecules
— based on hexagonal rings but may have 5/7-carbon rings
— Cg, has spherical shape, simple
molecular structure (Buckminsterfullerene)
Properties of nanotube
— cylindrical fullerene with high length to diameter ratio
- High tensile strength (strong bonds)
- Conductivity (deloc. electrons)
What is a single layer of graphite called
graphene
What is metallic bonding?
Forces of attraction between delocalised electrons and nuclei of metal ions.
Describe properties of metals
- High melting/boiling points (strong forces of attraction)
- High density
- Good conductors of heat and electricity (delocalised electrons)
- Malleable, soft (layers of atoms can slide over each other whilst maintaining the attraction forces)
What are alloys? Why are they harder than pure metals?
Alloys are mixtures of metal with other elements (usually metals).Different sizes of atoms distorts the layers, so they can’t slide over each other,therefore alloys are harder than pure metals.
What are the limitations of the simple model?
There are no forces between spheres and atoms, molecules and ions are solid spheres — this is not true
What does the amount of energy needed to change state from solid to liquid or liquid to gas depend on?
The strength of the forces between the particles of the substance. The nature of the particles involved depends on the type of bonding and the structure of the substance. The stronger the forces between the particles,the higher the melting point and boiling point of the substance
A pure substance will melt or boil at...what compared to the mixture?
A fixed temperature.A mixture will melt over a range of temperatures.
What are the three states of matter?
Solid,liquid,gas
Describe the properties of noble gases. Discuss the trends in properties down the group.
Non-metals, colourless gases, low boiling points, unreactive (full outer shell; they don’t easily accept or lose electrons).The boiling point increases down the group, as the atoms get heavier.
What is the law of conservation of mass?
The law of conservation of mass states that no atoms are lost or made during a chemical reaction so the mass of the products equals the mass of the reactants.
Define relative atomic mass (RAM)
average mass of atoms of an element (including isotopes), relative to the mass of an atom of carbon-12
Define relative formula mass (RFM).
the sum of the relative atomic masses of all the atoms in a compound
What is the relative formula mass of:
[a] CaF2
[b] C6H12O6
[a]78
[b]180
![<p>[a]78</p><p>[b]180</p>](https://knowt-user-attachments.s3.amazonaws.com/a87d8ded-aa84-42d8-b6b3-a6e5cdd7fa38.png)
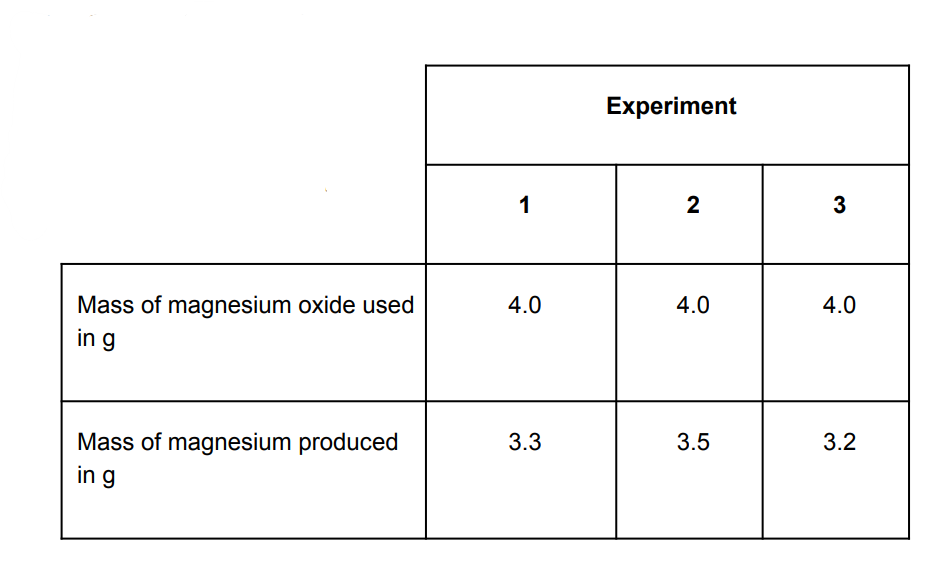
The experiment was repeated three times. Calculate the mean mass of magnesium produced and suggest how you could increase the precision of the results.
(33+3.5+3.2)/3=33
Measure to more decimal places or use a more sensitive balance /apparatus
What is Avogadro’s constant?
The number of atoms, molecules or ions in a mole of a given substance.
The value of the constant is 6.02 x 1023.
What is the formula that links mass, molecular mass and moles together
Mass(g)=Mr * Moles
What is the mass of 20 moles of calcium carbonate, CaCO,
2000g
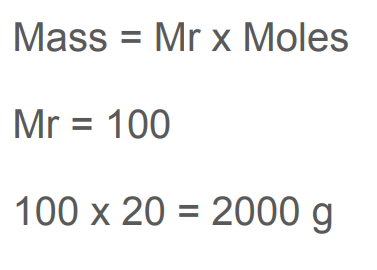
Calculate the amount of carbon dioxide in moles in 0.32 g of carbon dioxide.
Relative atomic masses (A): carbon = 12, oxygen = 16
0.007
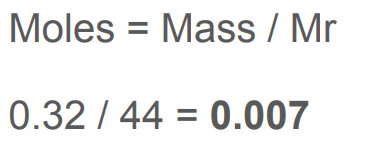
State what we mean by a limiting reactant in a chemical reaction
In a chemical reaction involving two reactants, it is common to use an excess of one of the reactants to ensure that all of the other reactant issued. The reactant that is completely used up is called the limiting reactant because it limits the amount of products formed.
Write down the two formulae that link concentration, mole/mass and volume together.
Concentration (g per dm?) = Mass (g)/Volume (dm?)
Concentration (mol per dm?) = nr of moles/volume (dm?)
What is the molar volume of a gas at room temperature and pressure?
1 mole of a gas at room temperature and pressure occupies 24 dm?
What is titration?
A technique for finding the concentration of a solution by reacting a known volume of this solution with a solution of known concentration.
Why is it not always possible to obtain the theoretical amount of product in a chemical reaction?
The reaction may not go to completion because It Is reversible.
Some of the product may be lost when it is separated from the reaction mixture.
Some of the reactants may react in ways different to the expected reaction (side reactions may occur).
How is the percentage yield of a product in a chemical reaction?

What is atom economy?
A measure of the amount of starting materials that end up as useful products.
It is a ratio of the relative formula mass of desired product(s) to the sum of the
relative formula masses of all reactants.