ATOMIC SPECTRA AND THE BOHR MODEL OF THE HYDROGEN ATOM | 1.2
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
Bohr orbit (n)
defines both the energy level and the position of the electron
characteristics of Energies of electrons
– quantized
– will not radiate
Energy of electron formula S
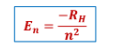
E =
energy of an electron in a particular orbit (in J)
n =
energy level or the principal quantum no. (n = 1, 2,
3...)
Rh =
Rydberg constant = 2.179 x 10^-18 J
lower n to higher n
Absorption Process?
higher n to lower n
Emission Process
n² = -Rh/En
energy level formula
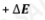
absorption unit?
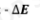
Emission unit
Line spectrum of hydrogen
Each line in the emission spectrum corresponds to a
particular allowed transition in a hydrogen atom
(400 to 700 nm)
Balmer series
falls within the
visible region
_______
atomic H emission spectrum series (low to high n)
lyman, balmer, paschen, brackett