chem atoms and orbitals test
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
atom
smallest unit of matter that still has the chemical properties of a larger sample
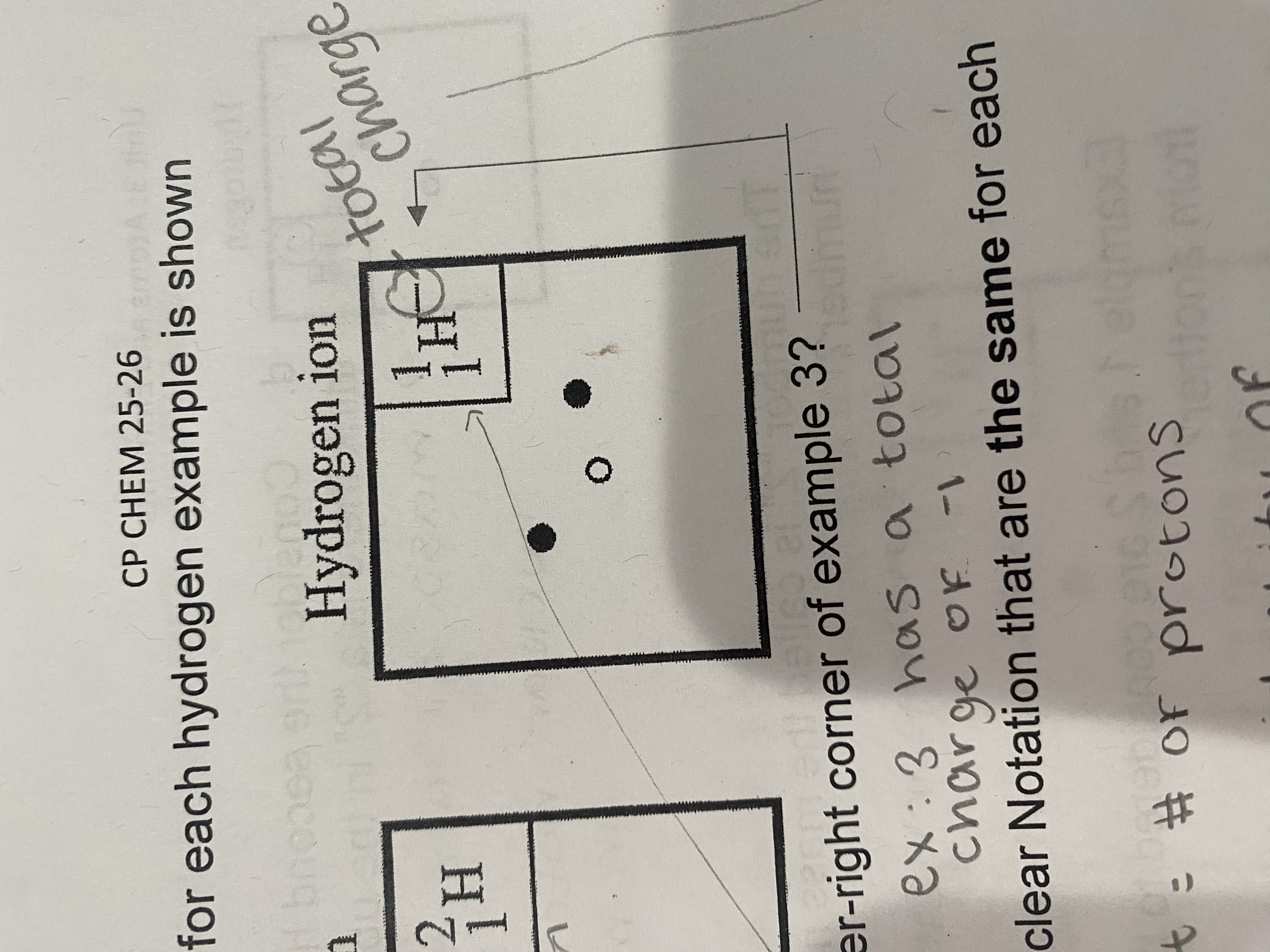
where do you put the total charge of an atom?
mass number
(protons + neutrons) number of particles in the nucleus
what makes isotopes differ?
they have different numbers of neutrons, ex: hydrogen with 1 neutron and hydrogen with 2 neutrons
rutherford model pros
you can easily count the number of electrons
rutherford model cons
you can’t see the particles in the nucleus well and there’s an unrealistic depiction of electron locations
what test did rutherford do?
he did the gold foil experiment, where he shot particles through gold foil, wondering what the inside of an atom looked like. some were deflected, and some went through, showing him that the atom is mostly empty with a heavy nucleus in the center.
element
different types of atoms, pure substances
atomic theory
all elements are made of atoms
atoms of the same element are identical
atoms of different elements can chemically combine
chemical reactions occur when atoms combine, separate, or rearrange with each other to create a different combination of atoms
what makes ions differ?
they are charged atoms with different numbers of electrons
requirements for isotopes
must have different numbers of neutrons (different mass number)
must have the same number of protons
weighted atomic mass
average based on how often we find the atom on Earth (natural abundance)
how to find atomic mass
turn percent abundance on Earth into a decimal by dividing by 100, then multiply answer by weight. at the end add all multiplied answers up.
valence shell
outermost shell where electrons are able to bon with other atoms
shell distance from nucleus
shells farther from the nucleus have a higher energy and are less stable
s orbital
spherical orbital shape, 3d area where electrons can be found
p orbital
looks like infinity signs, holds 6 electrons among the 3 p’s (Px, Py, and Pz), each holds 2
how to fill out periodic table for help
issue with orbital diagrams
you can’t see how many electrons are in each orbital
hund’s rule
each orbital holds a maxiumum of 2 electrons
electrons prefer to sit alone if possible
aufbau principle
orbitals having a lower energy will fill before higher energy orbitals (lower shells fill before higher shells)
orbital drawing
how to do box notation with p orbitals