Here....WK7: Blood Gases and pH Disorders
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
When we inhale what do we breathe in?
when we exhale, what comes out?
we inhale oxygen (O2) and exhale carbon dioxide (CO2)
Our bodies uses the oxygen and releases carbon dioxide as a waste product
What are blood gases?
When people refer to blood gases, what is the main type of test they are referring to?
What other blood gas test are there, and which is the gold standard?
Blood gases are
a group of tests that measure the levels of gases (O2, CO2, HCO3-) and the pH level (acidity/Basic) in your blood
When people refer to blood gases they mean artery blood gases, blood from artery.
venous blood gas (VBG), capillary blood gas (CBG).
ABG test is the gold standard for assessing lung function and overall acid/base status.
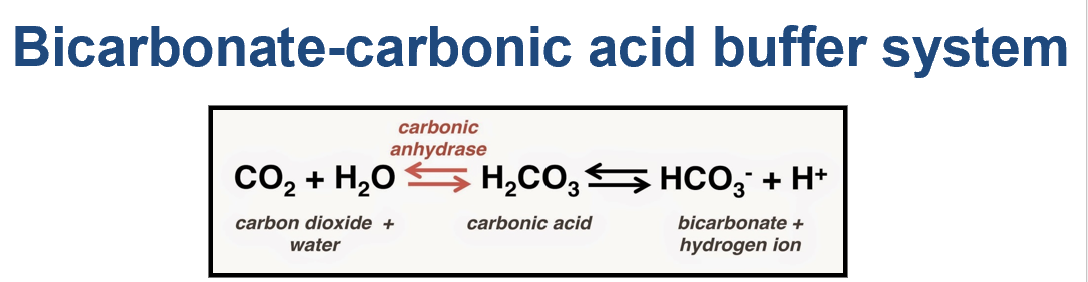
Bicarbonate formula
Carbonic acid formula
ABG stands for?
bicarbonate-carbonic acid system formula
HCO3- = Bicarbonate
H2CO3 = Carbonic Acid
ABG= Arterial Blood gas
CO2 + H2O → H2CO3 → HCO3 + H
Artery
Vein
Artery
Carries blood away from the heart
Arteries walls are thick
Have no valves and have high blood pressure
Oxygenated
except for pulmonary artery
Vein
Carries blood towards the heart
Veins walls are thin
Have valves and low blood pressure
Deoxygenated
except for pulmonary vein

The pH of the blood from an artery should be?
The pH of the blood from a vein should be?
Low Ph is what
High Ph is what
The pH of the blood from an artery should be:
7.35-7.45
The pH of the blood from a vein should be
7.31-7.41
Ph
acidic when it is low
Alkaline aka basic when it is high
What dos blood gas test tells us?
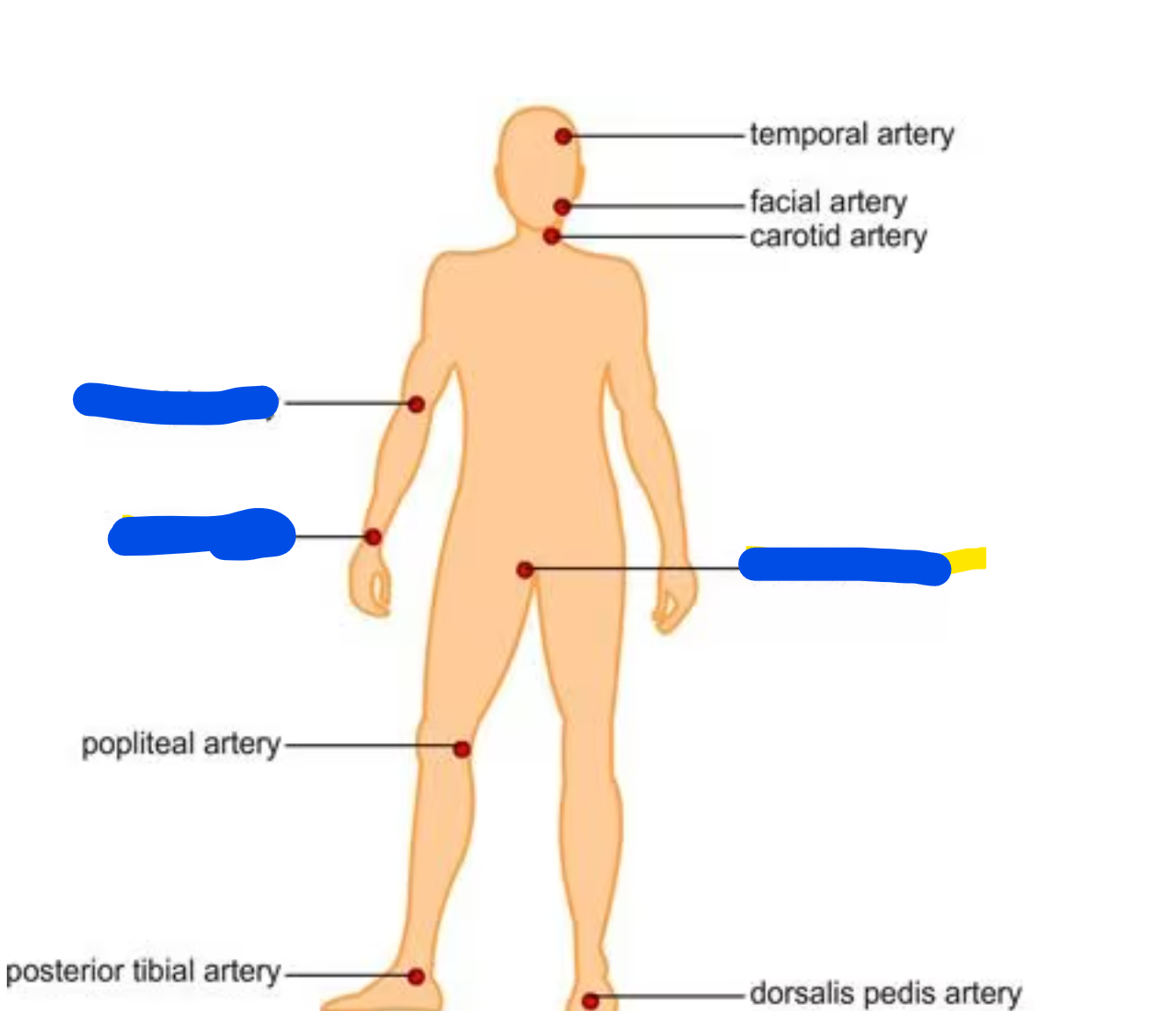
Where should we collect a blood gas sample from?
Should Blood gas test be STAT?
Blood gas test tell us how well your lungs are moving oxygen and carbon dioxide, and the acid/basic status of your entire body
We need a whole blood sample, preferable an arterial blood sample
Sites to acquire arterial blood are radial. femoral, or brachial
Blood Gas assessment should be performed STAT
What do blood gases test measure?
Arterial blood gas (ABG) test measures:
pH:
Measures the acidity or alkalinity of the blood.
pO2:
The partial pressure of oxygen, how much oxygen is present in the blood
% O2 Sat:
The percentage of oxygen saturation, measures the percentage of oxygen that your red blood cells are carrying
pCO2:
The partial pressure of carbon dioxide is how much carbon dioxide is in your blood.
HCO3 (Bicarbonate):
How much bicarbonate is in your blood, which is usually to test how well your kidney is doing and your acid-base balance.
Total CO2: The total amount of carbon dioxide in your blood.
CO2 + H2O → H2CO3 → HCO3 + H
Carbon dioxide + water= Carbonic acid= bicarbonate +hydrogen Ion
Base Excess:
Measures the amount of base (alkaline) in the blood
If negative, low on base
Sometimes measures
Lactic acid, Hgb, & other tests.
What 2 main form does oxygen exist in our blood?
Oxygen in your blood exists in two main forms:
Attached to Red Blood Cells (RBCs):
This is measured by oxygen saturation (% O2 Sat). Red blood cells have a protein called hemoglobin that binds to oxygen and carries it to your tissues and organs.
Dissolved in Blood Plasma:
This is measured by partial pressure of oxygen (pO2). Oxygen can be dissolved directly in the plasma, the liquid part of your blood, and is available for immediate use by your body.
what are blood thinners?
How to collect blood specimen for ABG?
What are some rules regarding ABG specimens?
What temperature do we test ABG and why?
Blood thinners are just anti-coagulant medications
ABG collection use heparin which is an anti-coagulant
We collect ABG specimen without exposing it to air (anaerobically) and put it on ice to keep the blood cells from changing the gas levels.
if the ABG is exposed to air, the oxygen and carbon dioxide levels in the sample will change, giving us wrong results.
We test the blood at body temperature (37 degrees Celsius) because that's how it is in your body.
The machine heats the blood to that temperature.
Some pre-analytical errors can be due to:
hint there are 5
What does ABG have to be on and what time can we test it?
1) Air bubbles:
Air bubbles: If air gets into the blood sample, it increases the oxygen (pO2), pH,
Also decreases the carbon dioxide (pCO2) levels, making the test results wrong
2) Clot
Can not run clotted whole blood on instrumentation since the machine cannot read it
3) Glycolysis:
"The blood cells keep using oxygen and making carbon dioxide even after the sample is taken.
This decreases the pH and oxygen levels
Increases the carbon dioxide levels
4) Temperature:
pH is temperature dependent so the temperature can change the Ph levels
5) Mixing:
We have to gently turn the sample upside down and back a few times to mix it, don't shake it.
Ice and Time
ABG usually comes on ice, and must be run w/in 30 min
Ranges for
pH
pO2
O2 saturation
pCO2
HCO3-
pH
7.35-7.45
pO2
80-100 mmHg
O2 Saturation
greater or equal to 95%
pCO2
35-45 mmHg
HCO3-
22-26 mEq/L
Do not need to know the rest, just these
What is one electrochemistry test and how do we use it?
What is one machine that allows us to measure the hemoglobin concentration?
Electrochemistry test includes (there are many more as well)
Ion selective electrodes
which is a machine that uses special sensors that measure the amounts of different ions (like hydrogen ions) in the blood.
Hemoglobin Concentration
Co-oximeters are spectrophotometers that shines light through the blood to measure the different types of hemoglobin (the stuff that carries oxygen)
What 3 gases do the machines directly measure?
what does the machine then calculate?
Machines usually directly measure
pH
Oxygen
carbon dioxide \
The machine then calculates other values like bicarbonate, base excess, and Oxygen Saturation aka % O2 Saturation, which is how much oxygen your red blood cells are carrying."
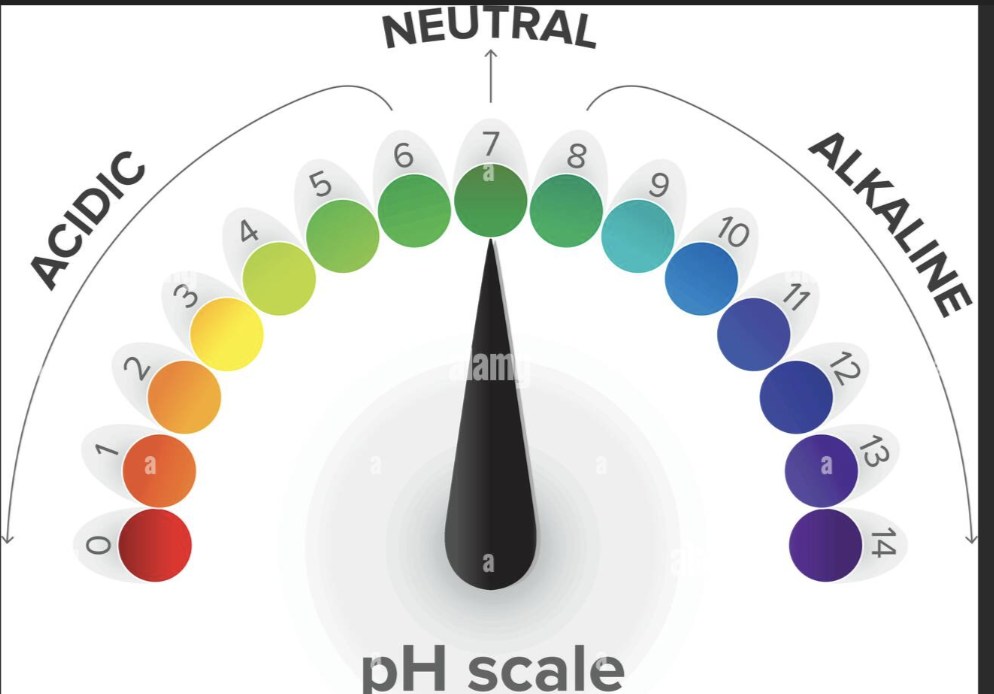
Acidic solution means what
Basic solution means what
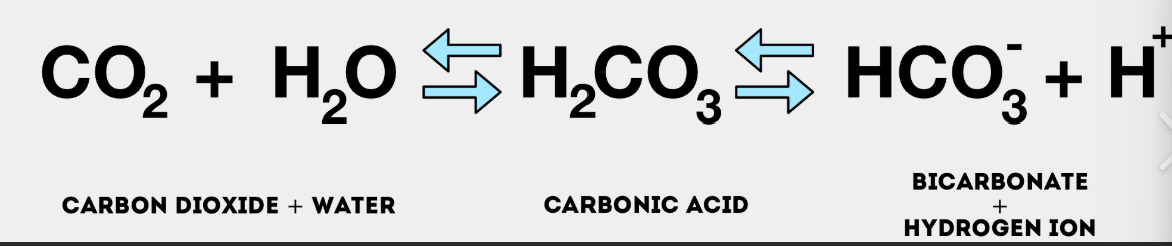
What is the bicarbonate-carbonic acid system?
what does it help do?
More H+ ions: The solution is acidic (pH is low).
Fewer H+ ions: The solution is basic (pH is high).
CO2 + H2O → H2CO3 → HCO3- + H+
The bicarbonate-carbonic acid system acts as a buffer to keep your body's pH level within a healthy range.
It helps neutralize excess acids or bases to maintain that balance
what is the bicarbonate-carbonic acid system formula?
is it reversible?
What happens to the formula when there is too much acid?
What happens when there is too much base?
At a normal pH of 7.45 the ratio of bicarbonate to carbonic acid is
is reversible:
CO2 + H2O → H2CO3 → HCO3- + H+
When there's too much acid (too many H+ ions) in your blood, bicarbonate (HCO3-) acts like a sponge to soak up those extra H+ ions, forming carbonic acid (H2CO3)
HCO3− + H+→ H2CO3
When there's too much base (too few H+ ions) in your blood, carbonic acid (H2CO3) can release H+ ions to balance things out. This helps neutralize the excess base
H2CO3 → H+ + HCO3−
20:1
is 20 bicarbonates to 1 carbonic acid
Define hypoventilation
Define hyperventilation
What does base excess show?
What does oxygen saturation tell us?
High carbon dioxide means you're not breathing enough (hypoventilation)
low carbon dioxide means you're breathing too much (hyperventilation) since you are exhaling all of the CO2
Base excess shows if there's too much or too little base in the blood:
Too little base (deficit) means too much acid (acidosis),
Too much base (excess) means too little acid (alkalosis)
O2 Saturation
Oxygen Saturation aka how much oxygen your blood is carrying, can be calculated using pH and oxygen levels.
To get a more accurate reading you can figure out the hemoglobin levels
What 3 main system does our body uses to have our blood gases balances?
Your body keeps your blood gases balanced using three main systems:
Chemical Buffers which are chemicals in the blood (buffers) that soak up extra acids or bases, those chemicals include
bicarbonate aka HCO3-
Phosphate aka HPO4^2-
Protein-like hemoglobin
Respiratory system
hypoventilation (breathing slowly)
Hyperventilation (fast breathing)
Renal aka kidney system by removing or keeping acid/base
H2O + CO2 « H2CO3 « H+ + HCO3-
why is this equation so important?
3 main reasons
H2CO3 disassociates into CO2 and H2O , this allows your lungs to get rid of carbon dioxide and acquire water
Changes in carbon dioxide changes your breathing (ventilation aka respiratory) rate,
your kidneys can change the amount of bicarbonate (HCO3-)
Why are phosphate buffers important?
where does phosphate buffer help out at?
Why are protein buffers important?
Name one protein buffer and how does it help.
Phosphate Buffers:
Phosphate Buffers help maintain pH balance by reacting with excess acids or bases. This forms compounds that only slightly change the pH, thus minimizing major pH shifts.
Phosphate buffers are effective in the renal tubules (the tiny tubes in the kidneys)
Protein Buffers:
Protein buffers, which includes hemoglobin and other proteins, help maintain pH both inside and outside of cells.
Protein buffers are the most abundant buffers in your body.
Hemoglobin is a protein buffer and thus, hemoglobin in red blood cells maintains blood pH by binding to hydrogen ions (H+).
Hemoglobin Buffering System is what
What happens when your cells use oxygen?
What does most of the carbon dioxide turn into inside the RBCs?
What causes bicarbonate (HCO3-) to diffuse out into the plasma?
What is the Chloride Shift and why does it happen?
Why do H+ ions remain inside the RBCs?
Hemoglobin Buffering System
H2O + CO2 « H2CO3 « H+ + HCO3-
When your cells use oxygen, they make carbon dioxide and then RBC comes by and picks it up.
Most of this carbon dioxide turns into carbonic acid, which splits into hydrogen ions and bicarbonate.
The increase in bicarbonate (HCO3-) inside RBCs causes it to diffuse out into the plasma.
Chloride Shift: To maintain electrical neutrality, chloride ions (Cl-) move into the RBCs to replace the bicarbonate ions that left.
The H+ ions remain inside the RBCs. They don't come out because their role is to be neutralized by binding to hemoglobin and other intracellular buffers.
This helps prevent changes in pH inside the RBC
Hemoglobin Buffering system for your Lungs; what are the steps
What happens when CO2 is not removed from the blood and is piled up instead?
H2O + CO2 « H2CO3 « H+ + HCO3-
Hemoglobin Buffering system for your Lungs
Oxygen (O2) from the lungs diffuses into the blood and binds to hemoglobin (forming oxyhemoglobin, O2Hb).
As oxygen binds to hemoglobin, hydrogen ions (H+) that were previously attached to hemoglobin are released
These H+ ions then combine with bicarbonate ions (HCO3-) in the blood to form carbonic acid (H2CO3)
Carbonic acid (H2CO3) quickly breaks down into water (H2O) and carbon dioxide (CO2)
The carbon dioxide (CO2) diffuses into the alveoli (tiny air sacs in the lungs) and is then exhaled out of the body
If CO2 is not removed from the blood, it piles up. The piled-up CO2 can then react with water to form more carbonic acid (H2CO3), which dissociates into more H+ ions and bicarbonate (HCO3-).
This increase in hydrogen ions (H+) leads to a condition called acidosis, where the blood becomes too acidic.
What is the second line of defense?
what does this second line of defense control?
What changes our breathing state?
When do you breathe faster?
When do u breathe slower?
H2O + CO2 « H2CO3 « H+ + HCO3-
Gasses in your blood: Respiratory system
Your lungs control carbon dioxide levels and are the second line of defense.
Chemoreceptors aka sensors in your brain changes your breathing rate
If carbon dioxide is high (and pH is low), you breathe faster (hyperventilate).
breathing faster we exhale more CO2
By exhaling more CO₂, we reduce the amount of carbonic acid (H₂CO₃) in your blood.
This helps lower the concentration of hydrogen ions (H⁺) and increase the pH.
If carbon dioxide is low (and pH is high), you breathe slower (hypoventilate)
breathing slowly conserves CO2 and allows us to form more carbonic acid (H₂CO₃)
This increases the concentration of hydrogen ions (H⁺) and decreases the pH
What is your third line of defense?
How long does this third line of defense take?
How does this third line of defense work?
if it is to acid what do they do?
If it is to basic, what do they do?
H2O + CO2 « H2CO3 « H+ + HCO3-
Third line of defense is your kidneys:
Kidneys take hours to days to work and control bicarbonate levels.
If your blood is too acidic, they keep bicarbonate.
If it's too basic, they get rid of it.
Kidney’s tubule cells can also make new bicarbonate to increase pH
The kidney can also produce acids and ammonia (NH3).
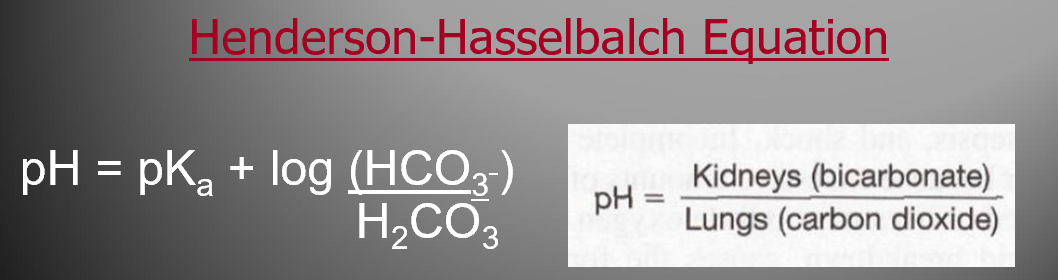
Kidney controls what
lungs controls what
What does the pH of your blood depend on?
Label Picture
H2O + CO2 « H2CO3 « H+ + HCO3-
Your kidneys control bicarbonate,
your lungs control carbon dioxide.
The pH of your blood depends on the balance between these two.
The Henderson- Hasselbalch equation shows how they're related (shown in the pic)
Basically, Kidney function divided by lung function."
What is the normal blood pH
Define Complete compensation
Define partial Compensation
1) Normal blood pH is 7.35 to 7.45.
Below 7.35 is acidosis/acidemia (too much acid)
Above 7.45 is alkalosis/alkalemia (too much base)
2) Complete Compensation
When the body's mechanisms (respiratory and renal systems) fully correct the pH imbalance and restores the pH to the normal range (7.35-7.45)
The ratio of bicarbonate (HCO3-) to carbonic acid (H2CO3) is brought back to the ideal 20:1
3) Partial Compensation
When the body's mechanisms have started to correct the pH imbalance but haven't fully restored the pH to the normal range yet
The pH is still outside the normal limits but is approaching normal as the body continues to adjust.
Respiratory Compensation
Metabolic Compensation
H2O + CO2 « H2CO3 « H+ + HCO3-
Respiratory Compensation
If the problem is in your body's metabolism (metabolic acidosis or alkalosis), your lungs (respiratory) help out by changing your breathing in order to fix the pH balance.
Breathing faster (hyperventilation):
If there's too much acid (low pH), you breathe faster to get rid of more CO2.
Helps in metabolic acidosis
Breathing slower (Hypoventilation):
If there's too much base (high pH), you breathe slower to keep more CO2.
Helps in metabolic alkalosis
Metabolic Compensation
If the problem is your respiratory aka lungs (lungs acidosis or alkalosis), then your kidney (metabolic) tries to fix it by changing the bicarbonate levels
If there's too much acid (low pH), your kidneys will reabsorb more bicarbonate (HCO3-) to raise the pH.
If there's too much base (high pH), kidneys get rid of bicarbonate (HCO3-) to lower the pH.
4 types of Acid-base problems, what are they?
H2O + CO2 « H2CO3 « H+ + HCO3-
4 types of Acid-Base Problems:
Metabolic Acidosis
Too much acid in stomach due to increase in acids or decrease of metabolic buffers like bicarbonate (HCO3-)
Lung will hyperventilate to decrease CO2 and kidney will retain buffers mainly bicarbonate and increasing excretion of acids
Anion gap is important for metabolic acidosis and a normal one is called hyperchloremia metabolic acidosis which is where chloride is a lot and bicarbonate not so much
Metabolic Alkalosis
too much base in stomach
Decrease of H+ and increase of bicarbonate (HCO3-) concentration, leading to increase in pH
The lungs will respond by hypoventilation, so CO2 increases in body in order to decrease the pH
Renal might happen by retaining acid and increasing the excretion of buffers mainly bicarbonate (HCO3-)
Respiratory Acidosis
Too much acid in your lungs by hypoventilation which leads to CO2 being piled up in your body which means more H+ and thus. lowers your pH
The kidney will respond by excreting acid, mainly H+ and retaining bicarbonate to increase pH
Respiratory Alkalosis
too much base in your lungs
May occur due to hyperventilation which leads to a decrease of pCO2 in the blood and thus decrease of H+ which leads to increase in pH
If this continues for a long time then the renal will start by retaining acid and excretion of buffer, mainly bicarbonate (HCO3-). This will lower the pH
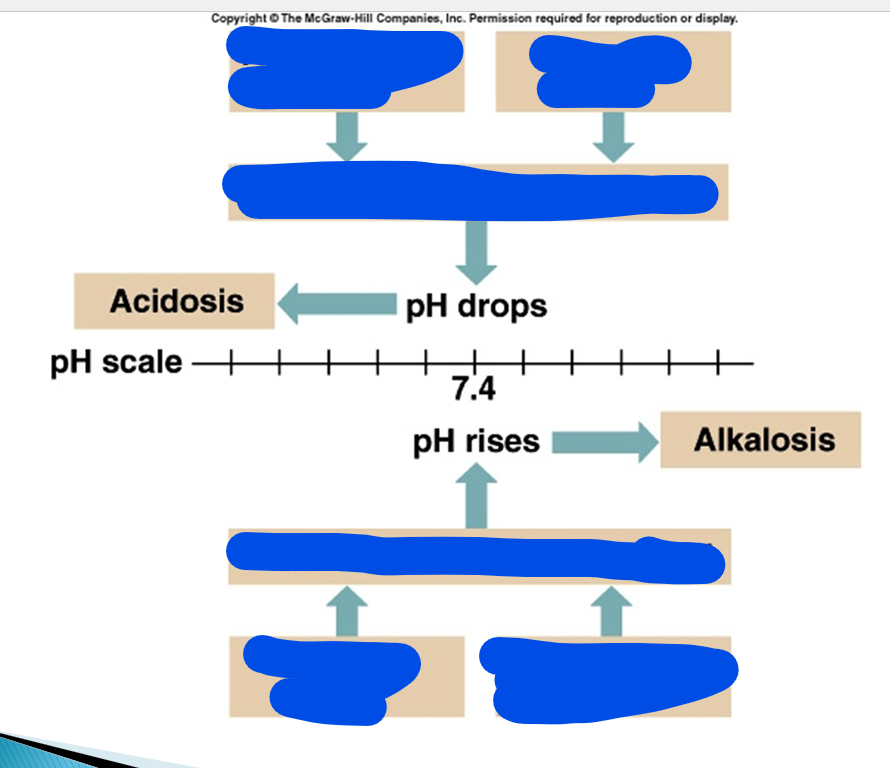
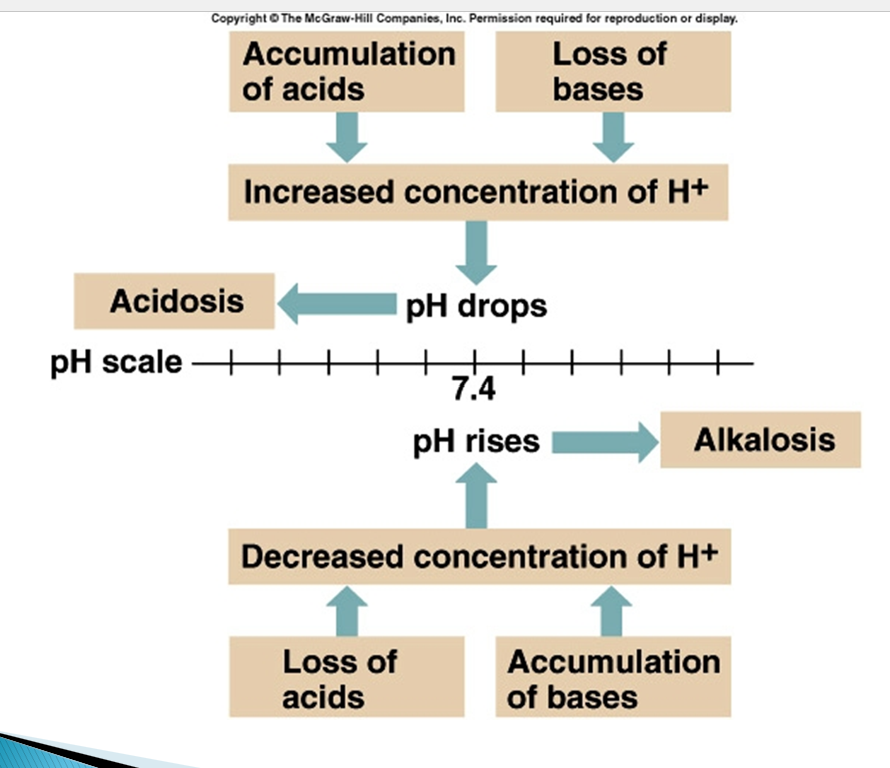
pH drops, is called what and what happens?
pH rises, is called what and what happens?
pH drops so is called acidosis which increases the number of H+ ions
which means the loss of bases and acids are piling up
pH increases so is called alkalosis which decrease in the number of H+ ions
which means the increase of bases
pCO2 is a
HCO3- is a
is a respiratory component
is a metabolic component
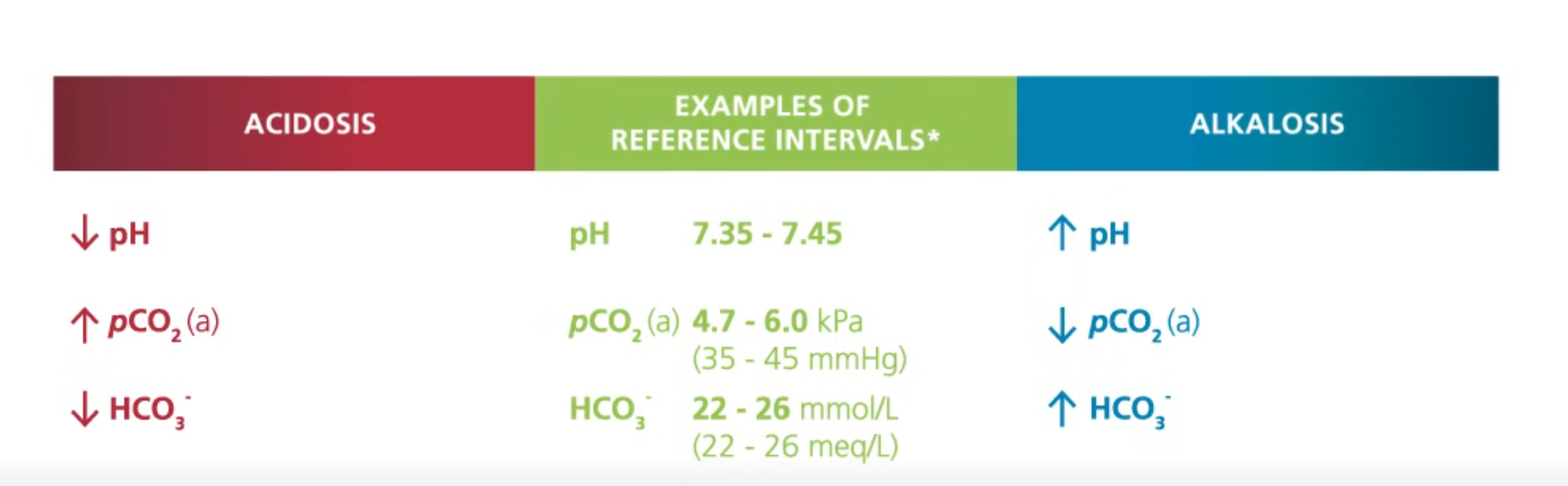
pH normal range is what
what happens if it is low or high
pCO2 normal range is what
what happens if it high or low
HCO3- normal range is what
what happens if the range is high or low
pH normal range is 7.35-7.45
low pH is acidic
high pH is basic
pCO2 normal range is 4.7-6.0 kPa aka 35-45 mmHg
increase in pCO2 means is acidic
Decrease in pCO2 means basic
HCO3- normal range is 22-26 mmol/L aka 22-26 meq/L
decrease in HCO3- is acid
increase in HCO3- is basic
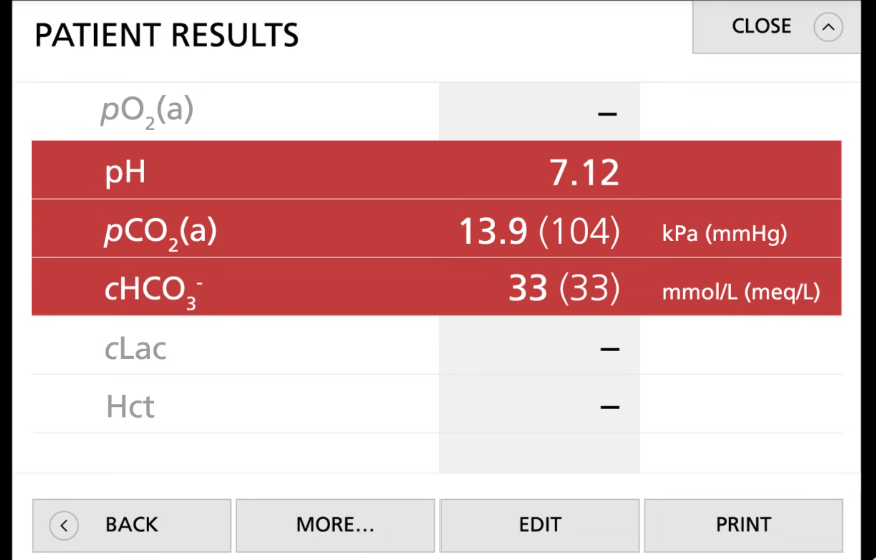
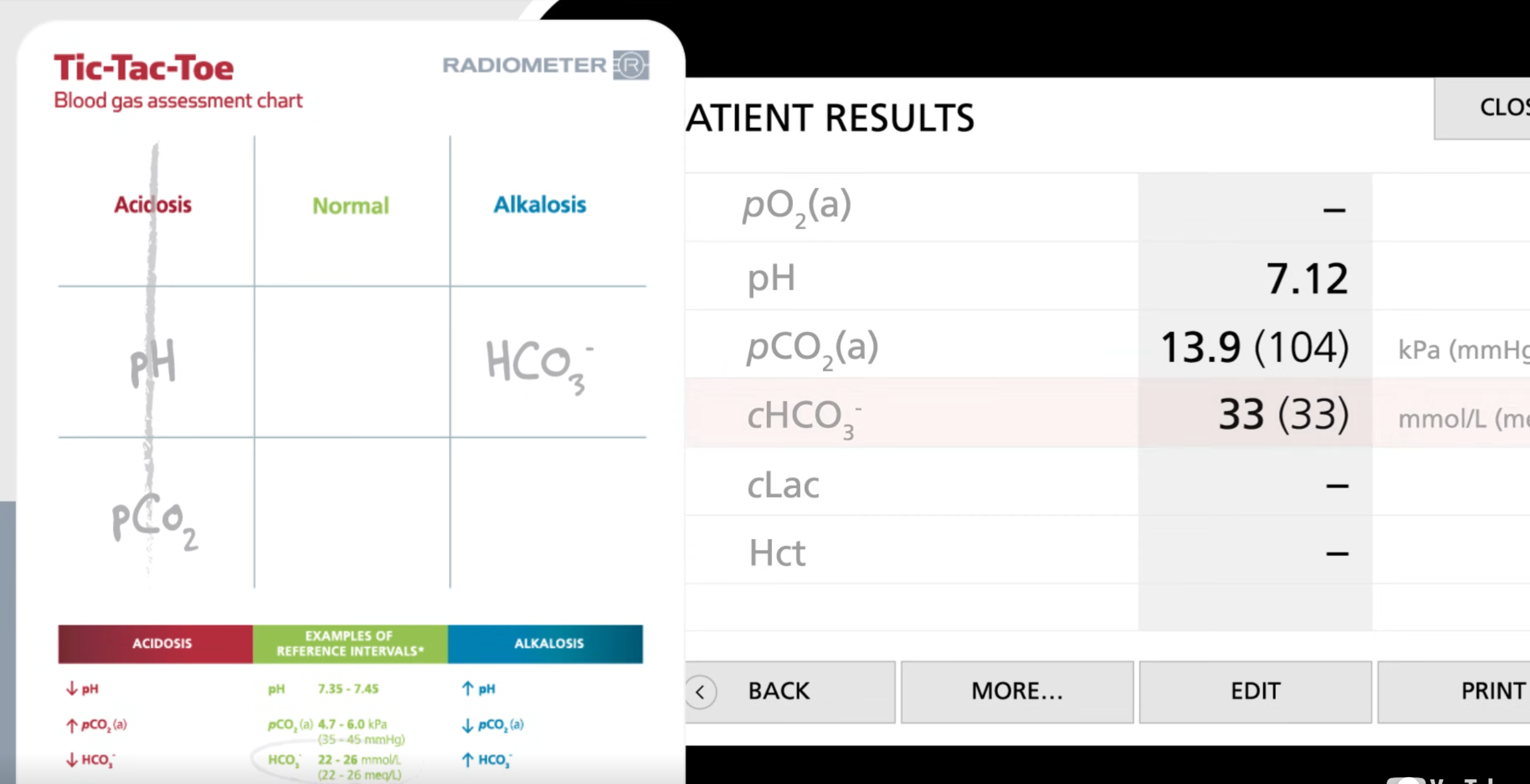
Problem: Plz use tik tac toe method
Patients has the following:
pH = 7.12
pCO2= 13.9 (104) KPa (mmHg)
cHCO3-= 33 (33) mmol/L (meq/L)
Since we got 3 in a row and pCO2 is respiratory, we can conclude this is respiratory acidosis
Then look for if the body tries to compensate for this:
if body did not do anything then the bicarbonate will be in the normal range
Since bicarbonate is not in reference range, the body is handling this situation
Define septic/sepsis
Define lactate
Define hematocrit
infected with microorganisms, especially harmful bacteria.
Lactate, also known as lactic acid, is a substance produced in the body during intense physical activity or when oxygen levels are low
Hematocrit (HCT) refers to the percentage of red blood cells (RBCs) in the total volume of blood.
basically, tells u how much RBC there are in the entire blood
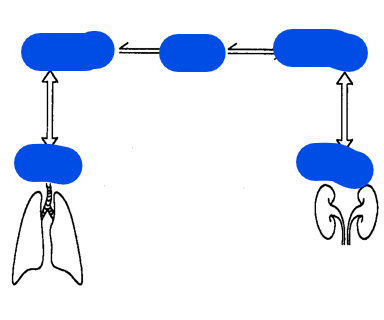
Metabolic aka what?
what does metabolic manage?
Respiratory aka what?
what does respiratory manage?
What balances what?
Metabolic aka Kidneys manages bicarbonate (HCO3-)
which controls the body's base aka alkaline levels
bicarbonate (HCO₃⁻) is a base because it can accept hydrogen ions
Respiratory aka Lungs manages carbonic acid (H2CO3) which is made from CO2
which controls the body’s acid levels
Carbon dioxide (CO₂) acts as an acid in aqueous solutions because it reacts with water to form carbonic acid (H₂CO₃), which can donate hydrogen ions (H⁺)
thus this makes CO2 part of the acid category
kidneys balance the base; lungs balance the acid.
Metabolic Acidosis
Metabolic alkalosis
Respiratory acidosis
Respiratory alkalosis
Metabolic acidosis is when your body/kidney has too much acid or not enough base (like bicarbonate)
body becomes to acidic
Metabolic alkalosis is when your body/kidney has too much base (like bicarbonate) or not enough acid.
body becomes to basic
Respiratory acidosis happens when the lungs can't remove enough carbon dioxide (CO₂)
Respiratory alkalosis is caused by the lungs getting rid of too much carbon dioxide (CO₂)
hyperventilation which is breathing to quickly
Metabolic Acidosis happens when
If bicarbonate (HCO3-) levels are low (below 22 mEq/L) then the pH of your blood will drop
thus becoming acidic
bicarb is a base
There is a 20:1 ratio Bicarb (HCO3-): Carbonic Acid (H2CO3-), when the bicarb drops then your blood becomes more acidic
bicarb aka bicarbonate
Metabolic Acidosis can be caused by:
severe diarrhea, kidney not working correctly which makes you lose bicarbonate
Piling up of to much acid like lactic acid from exercise or ketones from diabetes that your body can’t get rid of fast enough
Kidneys cannot remove the excess Hydrogen ion (H+)
H+ is an acid
Symptoms of metabolic acidosis includes:
Symptoms of metabolic acidosis includes:
Headache
Hyperventilation (rapid and deep breathing), aka Kussmaul breathing.
body is trying to get rid of acid by breathing out CO2.
Lethargy
tired
"Nausea (feel sick in stomach), vomiting, diarrhea"
Blood pressure drops
Muscle twitching
Warm and red skin, Vasodilation
skin may feel warm and look red because your blood vessels widen.
Vasodilation is when your blood vessels, particularly arteries, widen or relax.
How does one body try to compensate (fix) metabolic acidosis?
First mechanism is by the respiratory
Your lungs try to fix the problem by breathing faster and deeper to get rid of more carbon dioxide (CO2), which is an acid.
Renal excretion of hydrogen ions and reabsorption of bicarbonate
Gets rid of hydrogen ions and reabsorbs bicarbonate
Metabolic Alkalosis is what
Metabolic alkalosis happens when
Metabolic alkalosis happens due to
1) Metabolic Alkalosis is when your body becomes to basic
2) Blood has to much Bicarb (HCO3-), greater than 26 mEq/L
results in increase in pH, basic
Bicarb (HCO3-) is a base
3) Metabolic Alkalosis happens due to
Losing of stomach or other acidic fluid
vomiting makes you lose acid
Adding base to the body
Excessive use of alkaline substances
heavy use of antacids
antacids are medicine that neutralize acid in body
Decrease of base elimination
Endocrine disorder like Cushing syndrome which happens when Cushing syndrome happens when the body has too much of the hormone cortisol for a long time.
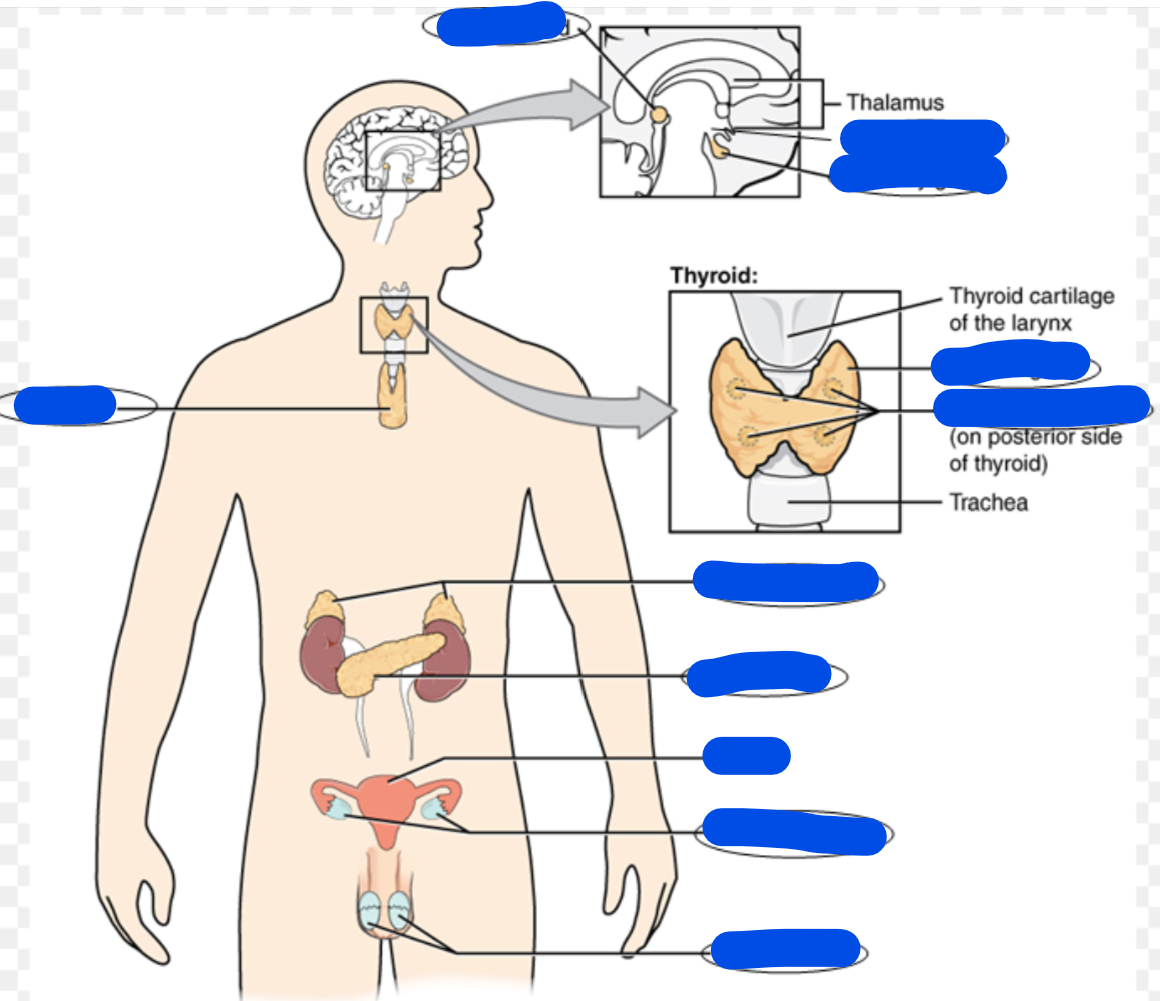
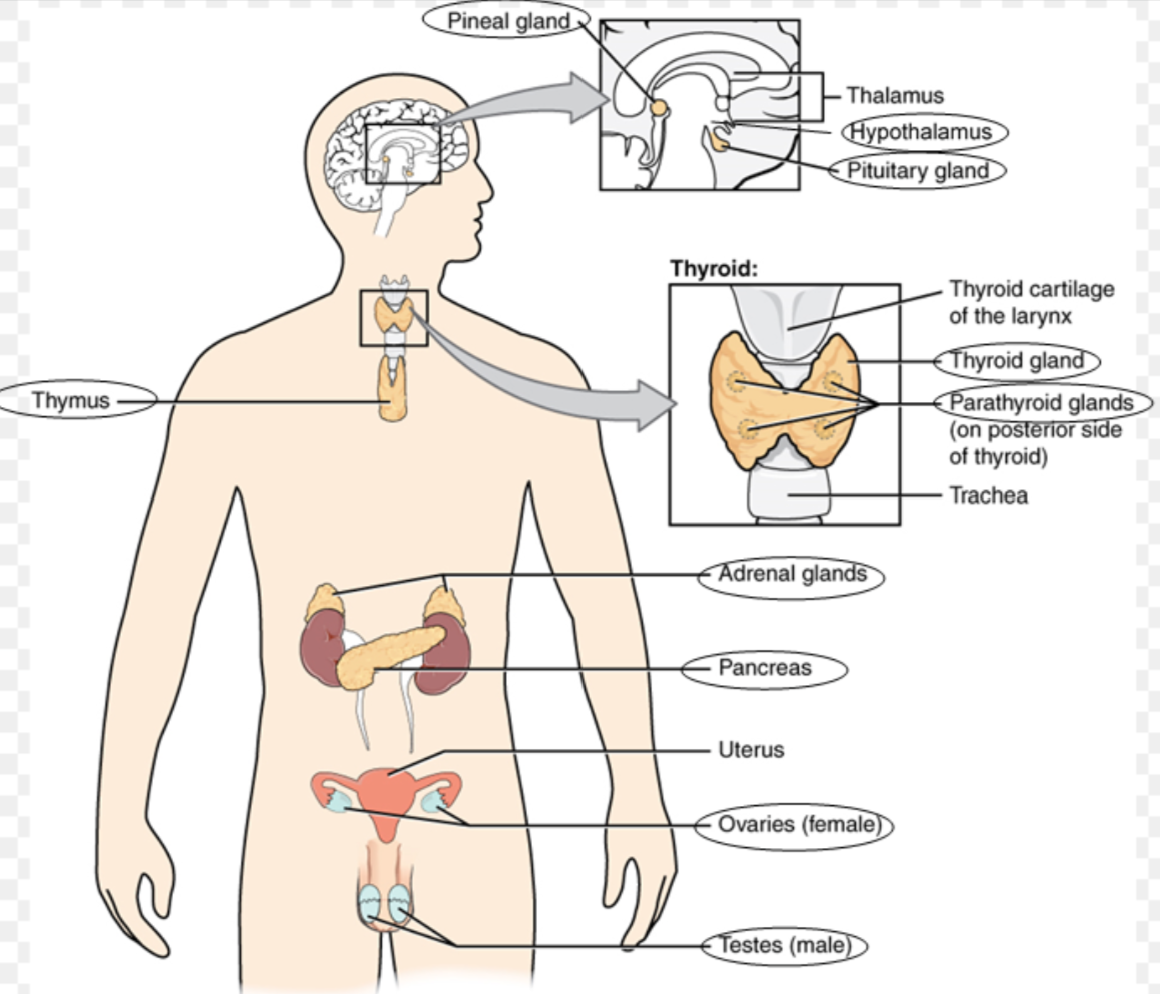
endocrine system define
Fill in the picture
The endocrine system is a network of glands and organs that produce and release hormones into the bloodstream
Symptoms of metabolic alkalosis
Respiration (breathing) becomes slow and shallow (not very deep)
Hyperactive reflexes or tetany
tetany is involuntary muscle contractions
often caused by electrolyte depletion
Atrial tachycardia
fast heart rate in upper chamber of heart
Dysrhythmias
not normal heart rhythms
Increased pH, increase in base, increase in HCO3-
HCO3- is bicarb
How does the body compensate (fix) metabolic alkalosis
Hypoventilation
Respiratory (lungs) try to fix it by breathing slower to keep more CO2 (an acid) in your body.
Hypoxia is a condition in which the body's tissues do not have enough oxygen
Alkalosis mainly occurs due to renal (kidney) problems, so the kidneys can't fix the problem by getting rid of extra Bicarb (HCO3-)
bicarb aka bicarbonate is a base
Respiratory acidosis is what
Respiratory acidosis happens due to
Too much carbon dioxide (CO2) which turns into too much carbonic acid
The excessive carbonic acid makes the blood more acidic, results in decrease of pH
Respiratory acidosis happens due to
Hypoventilation: This means slow or shallow breathing, leading to a buildup of carbon dioxide (CO₂) in the blood and making it more acidic
Chronic lung issues: Conditions like COPD or chronic bronchitis can reduce the lungs' ability to remove CO₂, contributing to hypoventilation
Respiratory depression: When the brain's signals to breathe slow down or weaken (from sedatives, brain injury, etc.), it also causes hypoventilation
Sings of Respiratory acidosis are
The body compensate (fixes) respiratory acidosis by
Air hunger: shortness of breath
lethargy (tired)
disorientated (confused/trouble thinking clearly)
Hypo-vent aka hypoventilation which is breathing to slowly which leads to CO2 build up
Hypoxia: low oxygen in the body
tremors (shaking/trembling)
convulsions (seizures)
1) the body tries to fix respiratory acidosis by
the kidney (first choice)
The kidneys try to fix it by getting rid of acid (hydrogen ions) and keeping more bicarb
Bicarb (HCO3-) is a base
Respiratory alkalosis is when
What is the most common body’s acid-base balance problem?
Respiratory alkalosis:
decrease of CO2 which leads to less carbonic dioxide (CO2), leading to less carbonic acid which makes the pH go up
Respiratory alkalosis is the most common body’s acid-base balance problem
Causes of respiratory alkalosis
causes of respiratory alkalosis includes
Hypoxemia (Low oxygen in the blood) which may be due to:
Pulmonary disease aka lung problems
Congestive heart disease aka heart problems
Severe anemia aka severe low iron
being at a High-altitude
Respiratory alkalosis may also be due to conditions that makes you breath fast includes:
Acute anxiety: When you're extremely anxious or stressed, it can trigger rapid breathing (hyperventilation).
Salicylate intoxication: Overdosing on certain medications like aspirin can mess with your breathing control and make you breathe faster.
Cirrhosis: A liver problem that can affect how your body controls breathing.
Gram-negative sepsis: A severe infection that can cause your body to go into overdrive, including faster breathing as a response.
Hyperventilation syndrome: A condition where someone chronically breathes too quickly, often due to stress or anxiety.
how does the body compensate (fix) Respiratory Alkalosis
The kidneys try to fix it by keeping acid (hydrogen ions) and getting rid of
bicarb (HCO3- which is a base)
Respiratory and metabolic acidosis are similar because
Respiratory and metabolic alkalosis are similar because
Both respiratory acidosis and metabolic acidosis result in the body becoming too acidic, but the key difference is where the problem starts:
Respiratory acidosis: This starts in the lungs, where they can't remove enough carbon dioxide (CO₂).
Metabolic acidosis: This begins in the body or kidneys, often due to too much acid production or loss of bicarbonate aka HCO3- a base (like in severe diarrhea), causing the body to become acidic
Both respiratory alkalosis and metabolic alkalosis involve the body becoming too alkaline (basic), but the difference lies in where the problem starts:
Respiratory alkalosis: The issue starts in the lungs, where too much carbon dioxide (CO₂) is being removed due to hyperventilation and thus, making the blood more basic
Metabolic alkalosis: The issue starts in the kidneys or body metabolism, often due to an excess of bicarbonate (HCO3-, a base) or a loss of acids (like through vomiting or diuretics)
Uncompensated define acidosis/alkalosis
Partially compensated acidosis/alkalosis
Fully compensated acidosis/alkalosis
1) Uncompensated: This means that the body hasn't made any attempt to fix the pH imbalance yet. The problem (acidosis or alkalosis) is happening, but there’s no action from the lungs or kidneys to try and balance it out.
2) Partially compensated: The body is trying to fix the pH imbalance, but it hasn't fully succeeded yet. For example, the lungs or kidneys are working to correct the issue, but the blood pH is still outside the normal range.
3) The body has successfully compensated for the problem, and the pH is now back to normal, but the underlying issue (like high CO₂ or bicarbonate levels) may still be present