3.2 Determining Empirical and Molecular Formulas
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
30 Terms
The elemental makeup of a compound
defines its chemical identity. Chemical formulas are the best way of representing this elemental makeup.
When a compounds formula is unknown
measuring the mass of each of its constituent elements is the first step in the process of determining the formula experimentally.
The Percent Mass of all elements in a substance add up to…
100%
Percent Composition
the percentage by mass of each element in the compound. EX: A gaseous compound composed solely of Carbon and Hydrogen. The percent composition of this compound is %H = mass H/Mass Compound X 100%. (see image attached).
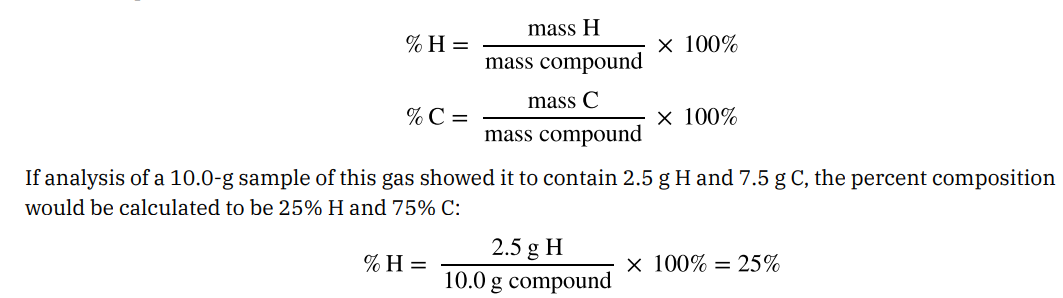
Percent Composition is useful for evaluating…
the relative abundance of a given element in different compounds of known formula.
An Object is 200g of brass. How many grams of Copper and Zinc does it contain? (Copper’s percent composition is 85% in brass)
0.85 × 200g = 170g of Copper (turned its percentage into a decimal to multiply) Mass of Zinc = Total Mass - Mass of Copper, 200g - 170g = 30g of Zinc, which makes up 15% of Brass since the other 85% is all Copper.
Percent Mass =
mass due to element/total mass X 100%
An object has a mass of 281g and it contains 43g of Al. What is the percent mass of Al in the object?
(Remember to use Percent Mass Formula) 43g of Al/281g of compound = 15% of Al. (Your calculator will show 0.149466 but you round up to 2 significant figures, giving you 15%).
How do you find % mass of an element in a compound (EX: %C in CO)
(number of atoms of element in compound) X (average atomic mass of element)/ formula mass of the compound X 100% (on your answer after dividing the numbers)
%C in CO?
1 × 12.01 amu/ 28.01 = 0.42877 × 100% = 42.88% mass composition of Carbon. If you subtract 42.88% of 100%, your answer 57.12% is the percent composition of Oxygen. (you can also verify your work by checking the periodic table. Oxygen has a higher amu than Carbon does, so this helps prove our answer right).
250g of a substance contains 38g of Oxygen. What is the % of O?
% of O = 38g/250g x 100 = 15%
The FORMULA for percent mass (# of atoms x amu)/ formula mass X 100) ONLY applies when…
A compound with a chemical formula is given. EX: C6H12O6 you can apply the formula for percent mass here since you have the chemical formula
When you have a percentage and want to find it in gram…
turn the percentage into a decimal, times it by the total grams. That gives you the percent in grams.
Percent composition can be applied…
In any circumstance. (Unlike percent mass). You can also use the Percent composition formula when asked for percent mass if you are not given a chemical compounds formula.
Steel contains 1.5% of Carbon. How many grams of Carbon are there in 675kg of steel?
Convert kilograms to grams, and multiply your answer by the percentage turned into a decimal. Your answer would be 1.01 × 10^4g
The FORMULA for percent mass when NOT given the chemical formula in a compound…
Mass due to element/total mass X 100%
Empirical Formula
The smallest whole number ratio of atoms of each element present in a compound.
EX: C3H8
This compounds formula is already in both empirical and molecular form as it cannot be simplified any further. The ratio is 3:8
The mol ratio of atoms of all elements in the compound…
need the mass that each element contributes to the compound.
4 Steps to Determine the Empirical Formula of a compound
Determine the mass (in grams) that each element contributes to the mass of a compound. (?/100%)
Use molar mass to determine the mole of each element in that mass compound (EX: going from grams to moles in that compound).
Determine the mol ratio between the elements by dividing by the smallest whole number ratio. (EX: You have the moles of Aluminum, Oxygen and Hydrogen. 1 Al, 2 O and 3 H, divide all of them by 1, since it is the smallest).
(SOMETIMES MAY NEED TO) Multiply all mole numbers by a constant to get the smallest whole number ratio. EX: One of your answers end in a decimal place, you want to end in a whole number. 1 and 1.5, multiply both by 2 to get two whole numbers, now you have 2 and 3.
When getting a decimal ending in .25, multiply by what to get a whole number?
4
When getting a decimal ending in .333 multiply by what to get a whole number?
3
When getting a decimal ending in .5, multiply by what to get a whole number?
2
When getting a decimal ending in .667, multiply by what to get a whole number?
3
When getting a decimal ending in .75, multiply by what to get a whole number?
4
When getting a decimal answer when trying to find the empirical formula of a compound
you can round up as long as the digits are not directly after the decimal. EX: you can round 1.025 but not 1.25
To find the molecular formula of a compound from it’s empirical formula, use…
n multiplied by the empirical formula EX: n=6 and the empirical formula CH2, multiply each subscript by 6 to get the molecular formula = C6H12
To find the molecular formula MASS…
n multiplied by empirical formula MASS
n =
n = molecular formula mass divided by empirical formula mass
n is equal to or bigger than…
1