Reaction Order Review
0.0(0)
0.0(0)
Card Sorting
1/5
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
6 Terms
1
New cards
Zero Order
Rate \= K
Units of K: M/s
Rate is independent of all concentrations
Units of K: M/s
Rate is independent of all concentrations
2
New cards
First Order
Rate \= k[A]
Rate \= k[B]
Units of K: 1/s or s^-1
Rate is directly proportional to one of the reactants independent of all other
Rate \= k[B]
Units of K: 1/s or s^-1
Rate is directly proportional to one of the reactants independent of all other
3
New cards
Second Order
Rate \= k[A]^2
Rate \= k[B]^2
Rate \= k[A][B]
Units of K: 1/Ms
Complex
Rate \= k[B]^2
Rate \= k[A][B]
Units of K: 1/Ms
Complex
4
New cards
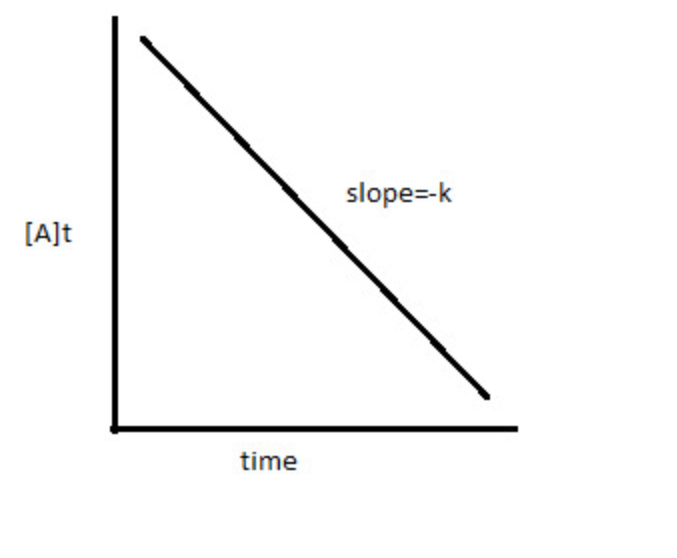
Zero Order
Rate \= K
\[A]t \= -Kt + [A]0
\[A]t vs. t
Half-Life: [A]0/2k
\[A]t \= -Kt + [A]0
\[A]t vs. t
Half-Life: [A]0/2k
5
New cards
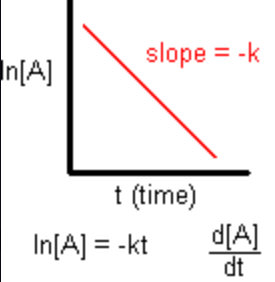
First Order
Rate \= k[A]
In[A] \= -kt + In[A]0
In[A]t vs. t
Half-Life: 0.693/k
In[A] \= -kt + In[A]0
In[A]t vs. t
Half-Life: 0.693/k
6
New cards
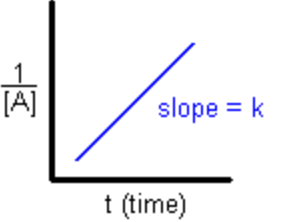
Second Order
Rate \= k[A]^2
1/[A] \= kt + 1/[A]0
1/[A]t vs. t
Half-Life: 1/k[A}0
1/[A] \= kt + 1/[A]0
1/[A]t vs. t
Half-Life: 1/k[A}0