Oxidation of Alcohols
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
oxidation in organic
whenever a carbon forms a bond with a more electronegative element
e.g oxygen
Primary alcohols are oxidised to…
aldehydes, which can be further oxidised to carboxylic acids.
Secondary alcohols are oxidised to…
ketones
During the oxidation of alcohols, Cr2O72- is reduced to Cr3+
Write the half equation for this reaction.
Cr2O72-+14H++6e- —→2Cr3++7H2O
A chemist added acidified potassium dichromate to an alcohol. The solution remained orange. Which possible class of alcohol does the chemist have?
Tertiary
A chemist has a bottle of unknown liquid. She knows it either contains a primary or a tertiary alcohol. How could she find out which one it was?
The chemist should add acidified potassium dichromate to the unknown liquid and heat the reaction mixture.
If the solution remains orange, this means an oxidation reaction has not taken place, so the bottle contains a tertiary alcohol. If the solution turns green, this means an oxidation reaction has taken place, so the bottle contains a primary alcohol.
How do we represent the oxidising agent in equations for oxidation reactions?
[O]
boiling point of ketones with the same number of carbons compared to alcohols have?
a lower boiling point.
what happens during reflux? (2)
Vapour condenses back into the reaction mixture
The reaction mixture is heated
what happens during distillation?(2)
The reaction mixture is heated
The product is separated from the reaction mixture
To separate aldehydes from the reaction mixture, we should use…
distillation
What forces do aldehydes experience…
Van der Waals forces
induced dipole-dipole forces
What method should we use to prevent the aldehyde leaving the reaction system?
reflux
To produce aldehydes, we use…
distillation
To produce carboxylic acids and ketones, we use…
reflux
how to extract the ketone from the reaction mixture?
distillation
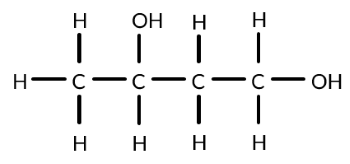
Butane-1,3-diol is oxidised using acidified potassium dichromate. Identify the structure of an organic product that could form when acidified potassium dichromate is not in excess.
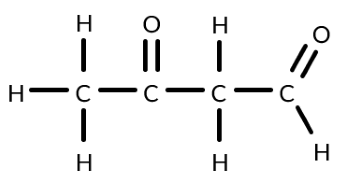
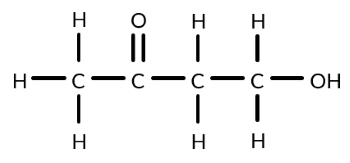
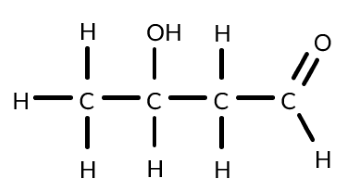
Draw a diagram of the equipment required to convert propan-2-ol to a ketone.
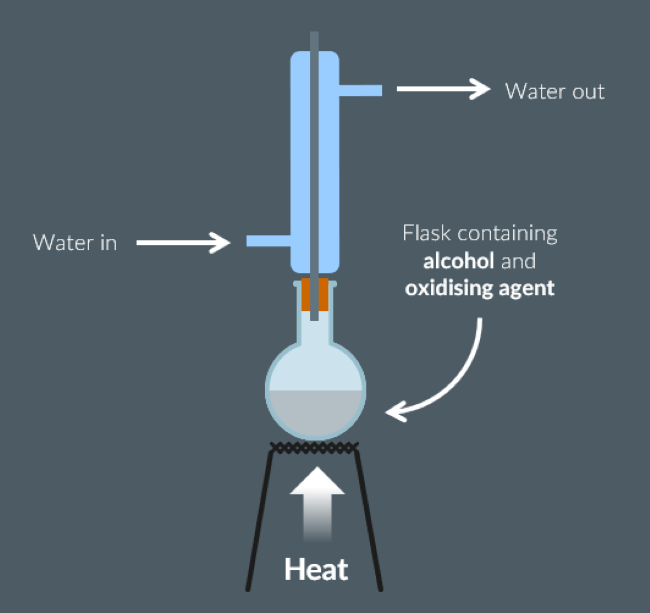
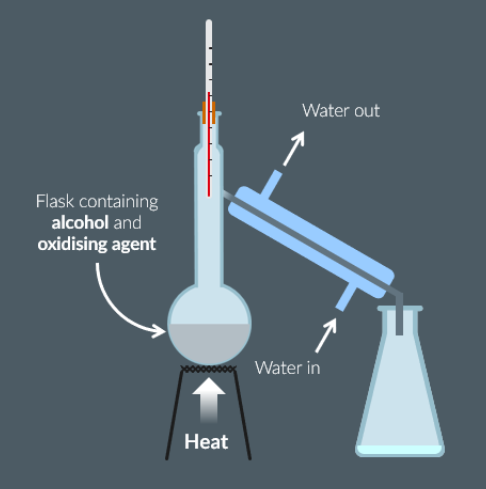
Draw a diagram of the equipment required to produce ethanal from ethanol.
If we add acidified potassium dichromate to a tertiary alcohol, the colour of the solution will be…
orange
To test whether an alcohol is primary or secondary, we can use…
Tollens’ reagent
what are two other reagents we can use to identify whether we have an aldehyde or ketone
Benedict’s solution and Fehling’s solution
Result when either Benedict’s solution or Fehling’s solution are added to an aldehyde?
red
Result when either Benedict’s solution or Fehling’s solution are added to a ketone?
no observable change