Chemistry: Structure 2 Unit 1
1/50
Earn XP
Description and Tags
Ionic bonding, ionic radius, ionization energy, electronegativity , and oxidation states
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
Why do atoms want to be chemically bonded?
Atoms bond to achieve the octet rule. Filled orbitals lead to atoms expending less energy.
What are ions?
Ions are atoms that have lost or gained a valence electron, developing a positive or negative charge.
What are cations?
Cations are positively charged ions. Usually, cations are metals because metal atoms only have a few valence electrons, and it is easier for atoms to lose a few electrons than gain many.
What are anions?
Anions are negatively charged ions. Usually, anions are non-metals because non-metal atoms are close to achieving the octet rule, and it is easier for atoms to gain a few electrons that lose many.
What are ionic bonds?
Ionic bonds are electrostatic forces of attraction between cations and anions.
What are metallic bonds?
Metallic bonds are electrostatic forces of attraction between cations and delocalized valence electrons.
What are covalent bonds?
Covalent bonds are electrostatic forces of attraction between nuclei and shared electrons of non-metal atoms.
How do you find an atom’s ion symbol?
Step 1: Find the atom on the periodic table and identify what group it is in.
Step 2: “This atom has # more/less electrons than it needs.”
Example: Calcium (Ca) is in group two. This atom has two more electrons than it needs. Therefore, it has a 2+ symbol: Ca2+
What is ionization?
Ionization is the process of atoms transferring valence electrons between each other to become ions.
What are ionic compounds?
Ionic compounds are compounds that form during the process of ionization.
They have a neutral charge of zero.
They have high melting points, high boiling points, and low volatility because of the strength of ionic bonds.
They can only conduct electricity when molten (l) or aqueous (aq) because the flow of electricity is limited when solid (s).
They are generally soluble in ionic or polar solvents (H2O) because the cations and anions are attracted to the oppositely charged molecules (Oxygen and Hydrogen).
They are brittle and shatter when a force that causes the movement of similarly charged ions toward each other is applied.
What are lattice structures?
Lattice structures are infinite three-dimensional crystalline structures built from oppositely charged ions. These structures make up ionic compounds.
How do you name an ionic compound?
Step 1: Write the name of the first element down. This first element identifies the metal ion.
Step 2: Write the name of the non-metal with the suffix -ide.
Example: (KBr) would be written as potassium bromide.
How do you write the formula for an ionic compound?
Step 1: Find the ion symbol for each atom. [Aluminum will form Al3+, and Oxygen will form O2-]
Step 2: Write the number of each ion’s charge above the ion:
3 2
Al O
Step 3: Cross-multiply these numbers.
Step 4: Write the final formula using subscripts and simplify if needed.
What are the two exceptions to the Aufbau principle?
Copper (Cu) and Chromium (Cr) break the Aufbau principle by transferring an electron from 4s into 3d to feel as stable as possible.
Why is it difficult to predict what ion transition metals and post-transition metals will form?
Transition metals and post-transition metals can lose different amounts of electrons from the d-orbital to form more than one type of ion.
How do you name transition metals and post-transition metals?
You can use the Stock system to name transition metals and post-transition metals. This system indicates the number of valence electrons the ion loses by placing a corresponding Roman numeral in parentheses directly after the name of the atom.
Example: Fe2+ is named Iron (II) ion. Fe3+, on the other hand, is named Iron (III) ion.
What does an oxidation state measure?
The oxidation state of an atom represents the atom's electron control/density when it is a simple element (not in a compound).
A (+) means the atom has lost electron control. This loss of electron control is the result of oxidation.
A (-) means the atom has gained electron control. This increase in electron control is the result of reduction.
What oxidation state do uncombined elements have?
Uncombined elements have an oxidation state of zero.
What oxidation state do ions have?
The oxidation state of an ion is equivalent to the charge of the ion.
What oxidation state do elements in a neutral compound have?
An element in a neutral compound has the same oxidation state as its ion symbol.
Hydrogen has an oxidation state of (+1) when with non-metals or (-1) when with metals and Boron (B).
Oxygen has an oxidation state of (-2) unless it is paired with peroxides such as Hydrogen Peroxide (H2O2) or Fluorine (F).
When paired with Hydrogen Peroxide, Oxygen has an oxidation state of (-1).
When paired with Flourine, Oxygen has an oxidation state of (+2).
Chlorine (Cl) has an oxidation state of (-1) unless it is paired with Oxygen or Fluorine, in which case it has an oxidation state of (+1).
What is the sum of all the oxidation states in a neutral compound?
In a neutral compound, all oxidation states must add up to zero.
What is the sum of all the oxidation states in a polyatomic ion?
In a polyatomic ion, the sum of all oxidation states must add up to the charge of the polyatomic ion.
What oxidation state does Flourine (F) have?
Fluorine always has an oxidation state of (-1). F is the most electronegative element.
What elements have unpredictable/various oxidation states?
The transition metals, N, P, S, Sn, and Pb have unpredictable/various oxidation states.
How do you find the oxidation state of a compound?
Step 1: Identify any atoms with a predictable oxidation state (Hydrogen or Oxygen) and assign them their value.
Step 2: Identify whether the compound is neutral or a polyatomic ion. Then, determine the sum of the oxidation states.
Step 3: Use algebra to determine the oxidation states of the unknown atoms.
What is a redox reaction?
A redox reaction is a chemical reaction in which the oxidation states of a species change.
What does lattice enthalpy measure?
Lattice enthalpy represents the strength of the ionic bonds in a particular ionic lattice.
What is the definition of enthalpy?
Enthalpy refers to the total energy stored in a substance.
What factors affect lattice enthalpy?
Ionic radius and ionic charge affect lattice enthalpy.
The lattice enthalpy value increases when the ionic charge of an ion increases.
The lattice enthalpy value decreases when the ionic radius of an ion increases.
What is an endothermic process?
An endothermic process is a process that requires the absorption of energy. Breaking an ionic bond is considered an endothermic process.
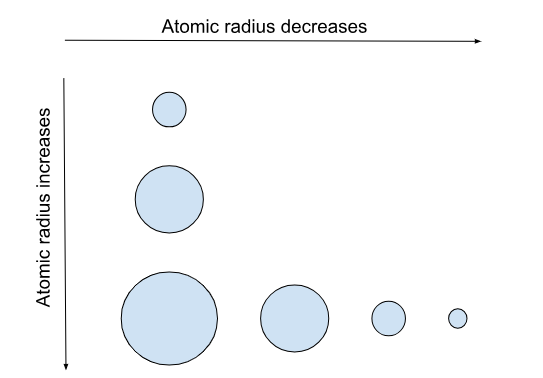
What is the atomic radius?
Atomic radius is the distance from an atom’s nucleus to the outer edge of its electron cloud.
Across a period, the atomic radius decreases; the atom gets smaller.
Think: “As you travel across a period, each element has one more proton than the prior element, and each element has the same amount of energy levels. Therefore, an element's effective nuclear charge is more powerful because there are more protons, and the nonvalence electrons’ shielding is less effective because there are fewer nonvalence electrons.
Shielding: Nonvalence electrons effectively repel valence electrons away from the attractive force of the protons in the nucleus through electron-electron repulsions. The more electrons an atom has, the less effective its nuclear charge is.
Electron-electron repulsions: Due to their similar charges, electrons orient themselves as far away from other electrons as possible, causing the electron cloud to expand.
Effective nuclear charge: the amount of positive, attractive force in an atom’s nucleus. The less powerful a nuclear charge is, the more spread out the electrons of an atom can become.
Down a group, the atomic radius increases; the atom gets larger.
Think: “As you travel down a group, the amount of energy levels increases, so there is a greater distance between the nucleus and the outermost orbital. This results in a larger atomic radius.”
Think: “ As you travel down a group, shielding increases, and the nuclear charge decreases, making the atomic radius larger.”
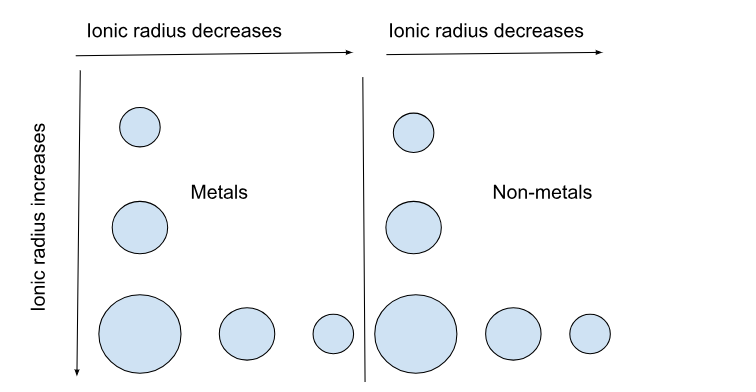
What is the ionic radius?
Ionic radius is the distance from an ion’s nucleus to the outer edge of its electron cloud.
The same trend as atomic radius applies once you divide the table into metal (cation) and nonmetal (anion) sections.
Cations are smaller than their neutral atom because the cations have a positive charge. A positive charge means a higher nuclear charge, meaning the radius is smaller. The reverse is true for anions because they gain electrons instead of giving them away.
What is ionization energy (IE)?
Ionization energy is the energy required to remove the first valence electron from a neutral, gaseous atom.
Think: If the effective nuclear charge of an atom increases across a period (making the radius smaller) and decreases down a group (making the radius bigger), it is more challenging to remove an electron from atoms higher in the group and further across the period.
The trend in ionization energy is the reverse of the trend in atomic radii.
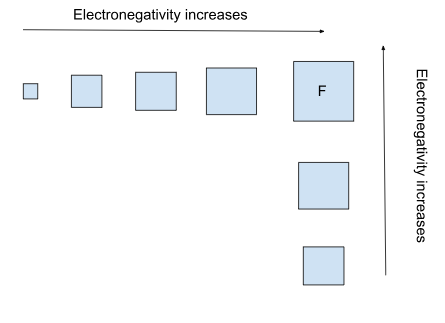
What is electronegativity?
Electronegativity represents the ability of an atom to attract a bonding pair of electrons in a covalent bond. Fluorine (F) is the most electronegative element. Cesium (Cs) is the least electronegative element.
Think: “The effective nuclear charge of an atom is more powerful as you go across a period, and the effective nuclear charge of an atom is more potent at the top of a group because there are fewer inner electrons and shielding. Therefore, the electronegativity of an atom increases as you go across a group and decreases as you go down a period.”
What is the Pauling Scale?
The Pauling Scale is used to determine if a compound is ionic or covalent by comparing the electronegativity values of atoms in a compound. Electronegativity ranges from 0.8 to 4.0.
The compound is likely ionic if the difference between atoms in a compound is greater than 1.8.
The compound is likely covalent if the difference between atoms in a compound is less than 1.8.
Exception: Beryllium fluoride (BeF2) and Beryllium chloride (BeCl2) are always covalent.
Where are the metal elements located in the periodic table?
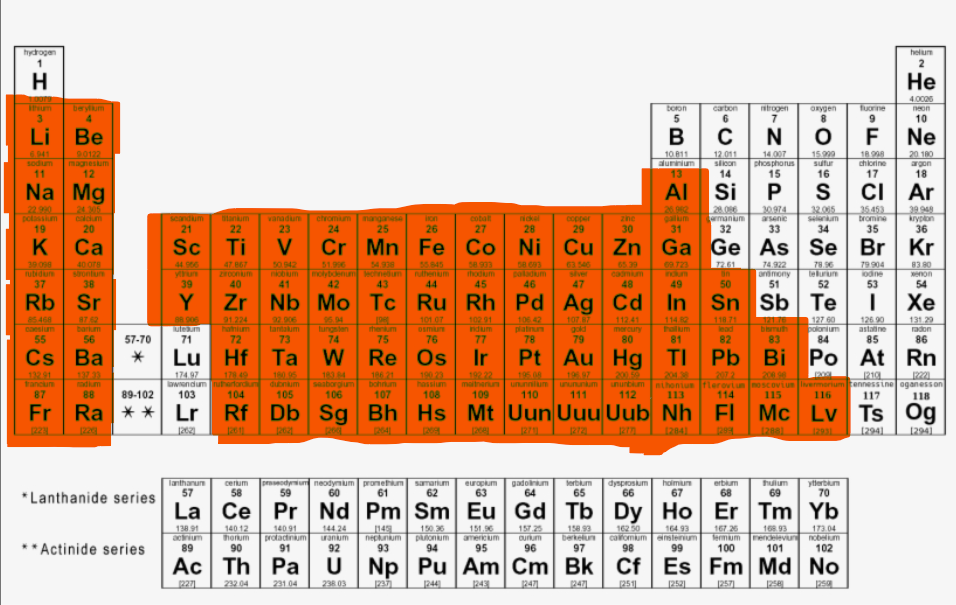
Where are the transition and post-transition metal elements located?
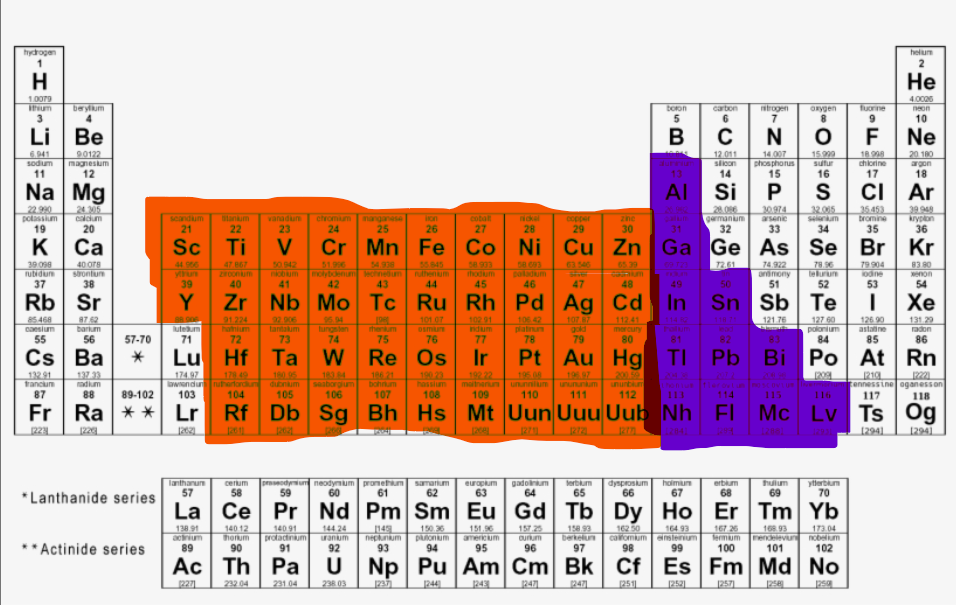
Where are the S-block elements located?
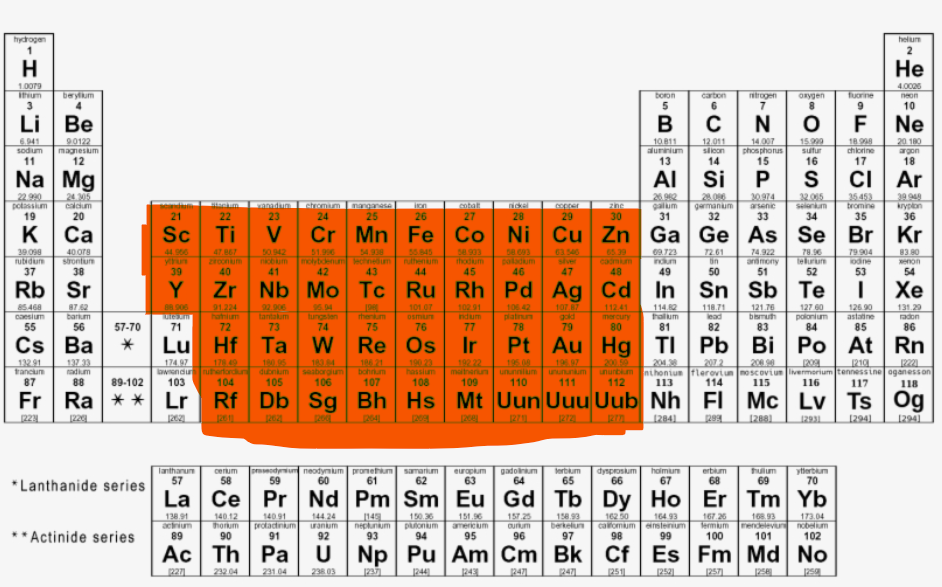
Where are the D-block elements located?
Where are the P-block elements located?
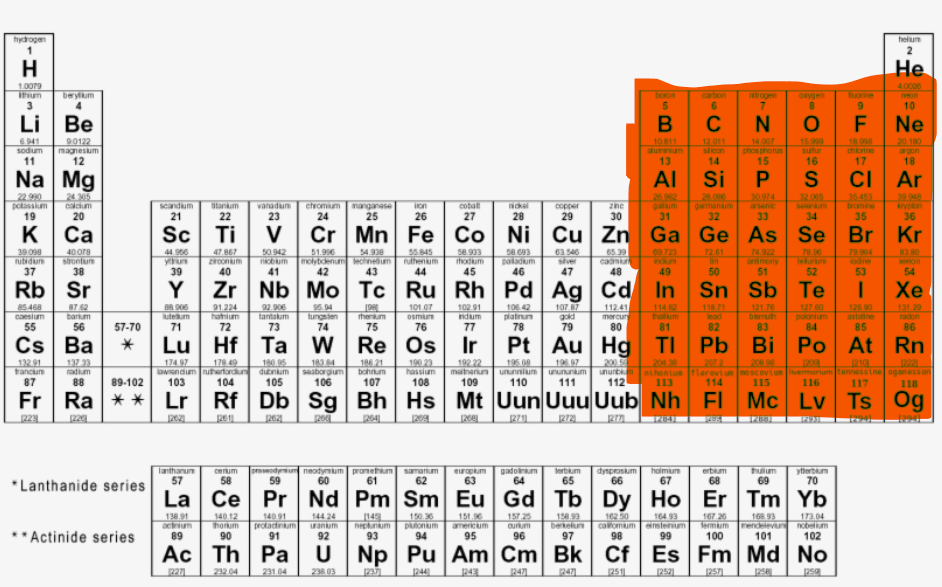
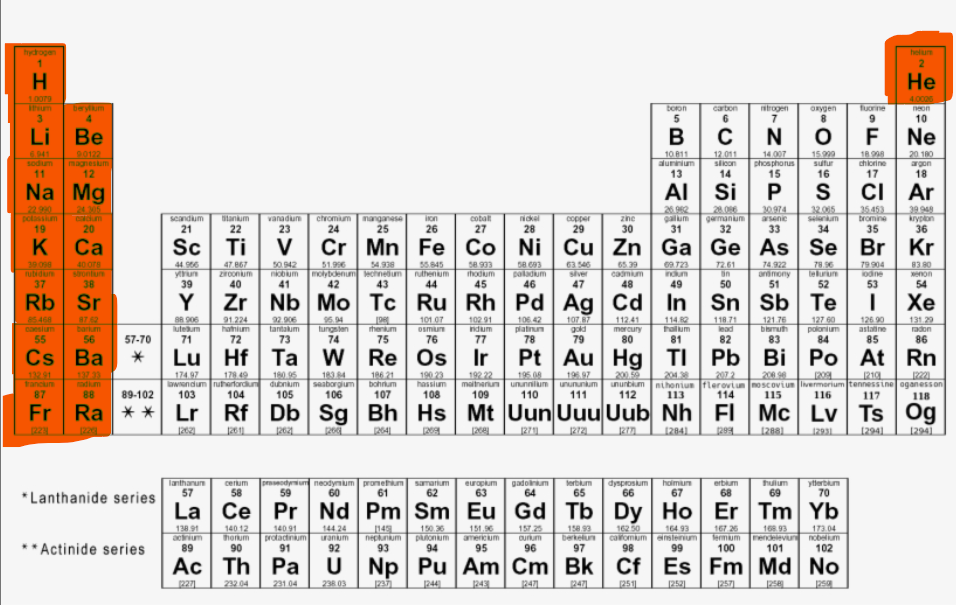
Where are the F-block elements located?
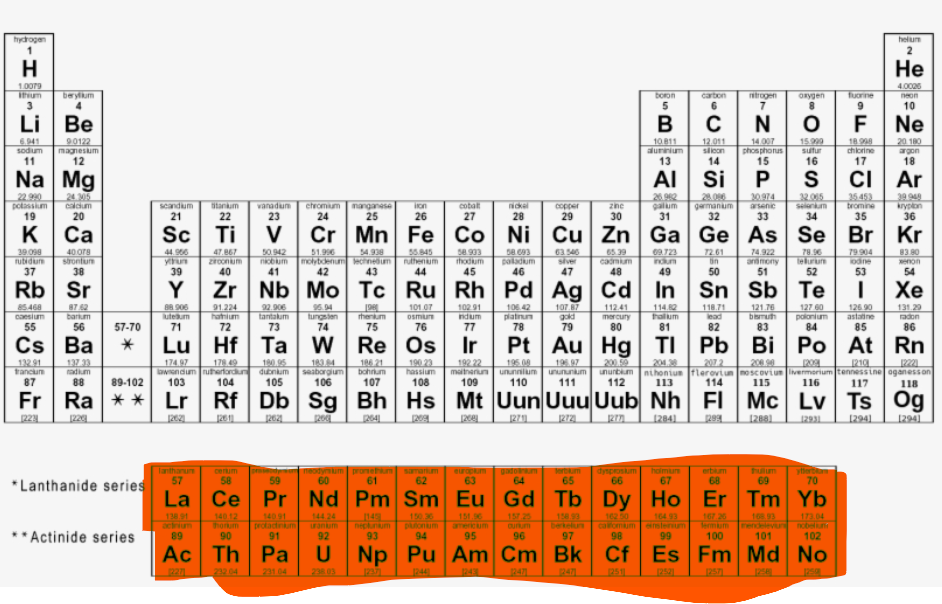
What is Nitrite?
NO2-
What is Nitrate?
NO3-
What is Hydroxide?
OH-
What is Hydrogen Carbonate?
HCO3-
What is Carbonate?
CO32-
What is Sulfite?
SO32-
What is Sulfate?
SO42-
What is Phosphite?
PO33-
What is Phosphate?
PO43-
What is Ammonium?
NH4+