20 - addition polymerisation
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
addition polymerisation
one of the most important addition reactions of alkenes which form the basis of the plastic industry
Addition polymerisation is the reaction in which many monomers containing at least one C-C double bond form long chains of polymers as the only product
Just like in other addition reactions of alkenes, the π-bond in each C-C bond breaks and then the monomers link together to form new C-C single bonds
polymer
a long-chain molecule that is made up of many repeating units
monomer
The small, reactive molecules that react together to form the polymer. they have the ability to form chemical bonds to at least two other monomer molecules.
a polymerisation reaction can be represented by
a general formula or by using displayed formulae
E.g. poly(ethene) and poly(chloroethene) (also known as PVC) are polymers made up of the ethene and chloroethene monomers respectively and are commonly used in making plastics
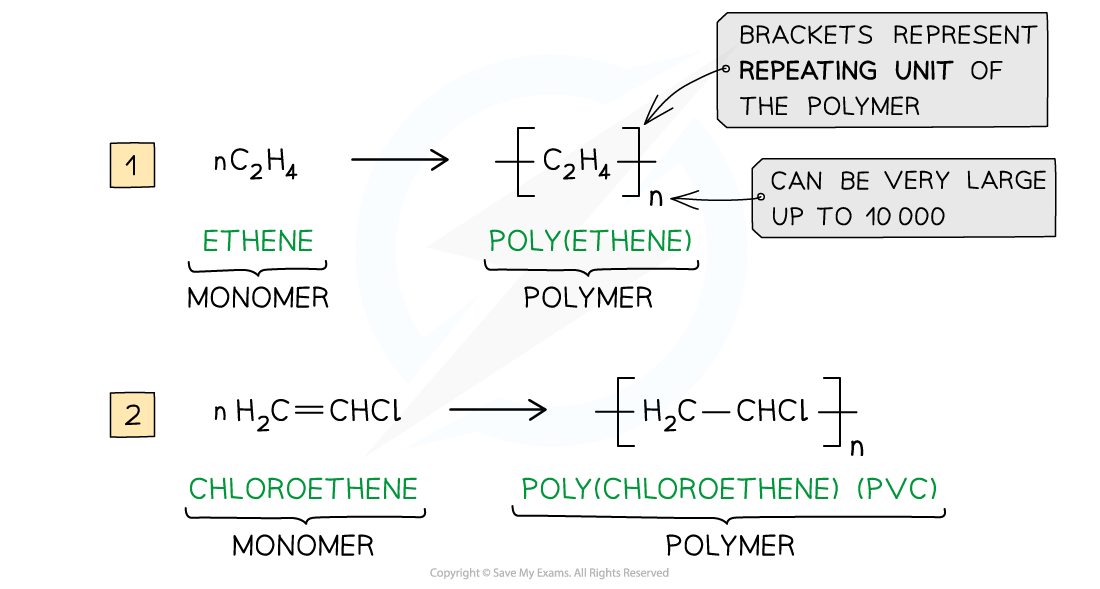

Just like any other addition reaction of alkenes, addition polymerisation gives only one product
a repeat unit
the smallest group of atoms that when connected one after the other make up the polymer chain
It is represented by square brackets in the displayed and general formula
In poly(alkenes) (such as poly(ethene)) and substituted poly(alkenes) (such as PVC) made of one type of monomer the repeating unit is the same as the monomer except that the C-C double bond is changed to a C-C single bond

examiner tips and tricks
The section of the polymer chain shown inside the square brackets by the structural or displayed formula is the repeat unit and not the monomer
The monomer is the same as the repeat unit except for that it has C=C bonds instead of C-C bonds
uses of Poly(ethene) (Polyethylene)
Use: Plastic bags, cling film, bottles, containers, insulation for wires.
Why: Flexible, lightweight, waterproof, chemically inert, cheap.
uses of Poly(propene) (Polypropylene)
Use: Crates, ropes, carpets, food containers, medical equipment.
Why: Stronger than poly(ethene), resistant to chemical and physical stress, lightweight.
uses of PVC – Poly(chloroethene)
Use: Window frames, pipes, waterproof clothing, vinyl flooring.
Why: Rigid (but can be made flexible with plasticisers), durable, water-resistant, good insulator.
uses of PTFE – Polytetrafluoroethene (Teflon)
Use: Non-stick coatings on cookware, electrical insulation, chemical containers.
Why: Very unreactive, high melting point, non-stick, good insulator.
uses of Polystyrene
Use: Packaging foam, disposable cups/plates, insulation, CD cases.
Why: Lightweight, insulating, shock-absorbent, rigid (in its hard form) or cushioning (in expanded form).
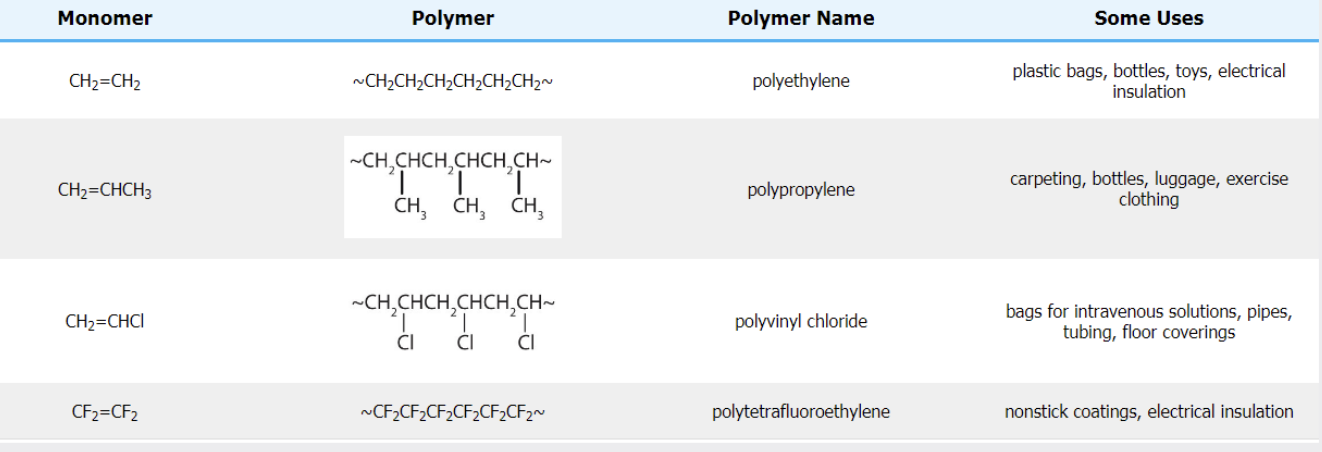
uses of common addition polymers
-
environmental issues with polymers/plastics
Poly(alkenes) are extremely important in everyday life, such as their use as plastics
However, the disposal of these polymers is problematic
Poly(alkenes) are very large alkane molecules which are unreactive and therefore do not undergo any chemical reactions; they are resistant to chemical attack
Due to their unreactivity, polymers are non-biodegradable and take up to hundreds of years to decompose when dumped in landfill sites
Throwing away poly(alkenes) therefore causes long-term pollution of the environment
they can cause a litter problem if disposed of carelessly and suitable places for landfill sites are difficult to find and space in landfill sites is wasted if it is filled with non-biodegradable polymers.
Burning the polymers results in harmful combustion products which again cause the pollution of the environment (releases co2 - a greenhouse gas which contributes to global warming -, toxic gases which need to be removed by scrubbers before they leave the chimney, hydrogen chloride and dioxins)
Animals can ingest plastic or get entangled in it.
Microplastics (tiny plastic fragments) are now found in oceans, soil, and even food.
Most plastics are made from crude oil, a non-renewable resource.
This adds to carbon emissions and depletes finite fossil fuel reserves.
recycling polymers/plastics
Recycling involves:
Melting the waste polymer
Forming the polymer into a new product
However waste must be clean and different polymers must be separated from each other first. This can be difficult and expensive to do.