chapter 6- mechanism of enzymes
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
what are the principles of enzyme catalysis?
covalent interactions
non-covalent interactions
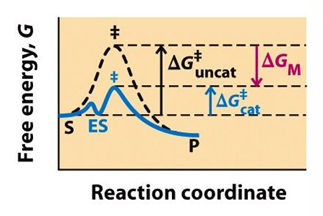
what is the difference between covalent and non-covalent interactions?
covalent interactions between enzyme and substrate may lower the energy of activation by providing an alternative, lower energy pathway
non-covalent interactions are used to establish binding interactions that contribute to catalysis and transient interactions that specifically stabilize the transition state
what happens to a substrate when there is no enzyme?
using the analogy of a stick
the stick is the substrate. it must bend into the transition state (a bent stick) before breaking into products
there is a high activation energy meaning that the reaction is slow and inefficient
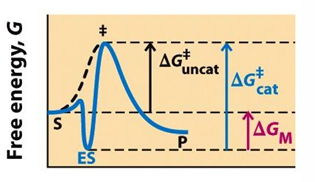
what happens when the enzyme is complementary to substrate?
an enzyme is tightly bound to the substrate using non-covalent interactions
the tight fit does not help the substrate reach the transition state
Instead of lowering activation energy, it will increase
what happens when an enzyme is complementary to transition state?
the enzyme will loosely bind to the substrate
it will allow the substrate to go the transition state as it is shaped to tightly bind to the TS, also using non-covalent interactions
his is faster and efficient and will lower the activation energy
what does a 10-fold increase correspond to?
5.7 kJ/mol of stabilization
what does a 100-fold correspond to?
it is 5.7kJ/mol * 2
so it is 11.4kJ/mol
what does a 1017 fold correspond to?
it is 5.7kJ/mol * 17
so it is 96.9kJ/mol
why are weak interaction important in catalysis?
energy available from a single weak interaction is 4-30 kJ/mol
so a few weak interactions that are position correctly can achieve significant catalysis
why do enzyme active sites exclude water?
water competes with substrate for hydrogen bonding and can disrupt the precise network of weak interactions
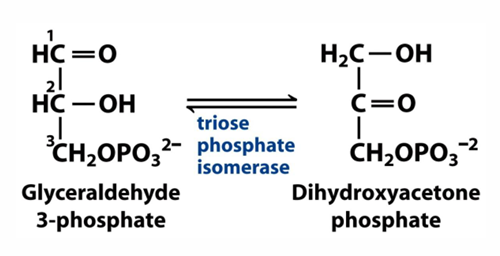
explain how this reaction relates to catalysis
in this reaction, the phosphoryl group plays a role in positioning the substrate for catalysis
these phosphoryl group interactions account for more than 80% of rate acceleration and help to discriminate between different substrates
what mechanisms (non-covalent interactions) help with catalysis?
entropy reduction → locks the substrate in place to be correctly oriented
desolvation of substrate → enzyme binding pushes out water allowing direct contact between enzyme and substrate
rearrangement of substrate → nudging it toward the transition state
conformation change in enzyme → change shape when substrate binds
where is the formation of an ES complex placed?
in proximity to reactive amino acid residues in the enzyme active site
what are the two major chemical modes of catalysis?
acid-base catalysis
covalent catalysis
how are amino acids residues important in
they are involved in catalysis
can help with the binding of substrates and transitions states
His is 6x more likely to be involved as its side chain can act as proton donor or acceptor
in acid-base catalysis, how is acceleration of a reaction achieved?
by catalytic transfer of a proton
what is the difference between general acid-base and specific acid-base catalysis?
general acid base:
proton transferring agents
often an amino acid side chain
specific acid-base
uses H+ or OH-
what are the two ways that a proton acceptor can assist reactions?
can cleave O-H, N-H, and even some C-H bonds by removing a proton
general base can participate in cleavage of other bonds involving carbon (C-N bonds) by generating the equivalent of OH- in neutral solution through removal of a proton from a molecule of water
how does metal ion catalysis contribute to catalysis?
they have a preference in geometry
can stabilize negative charges
lower pka of water molecules for nuc attacks
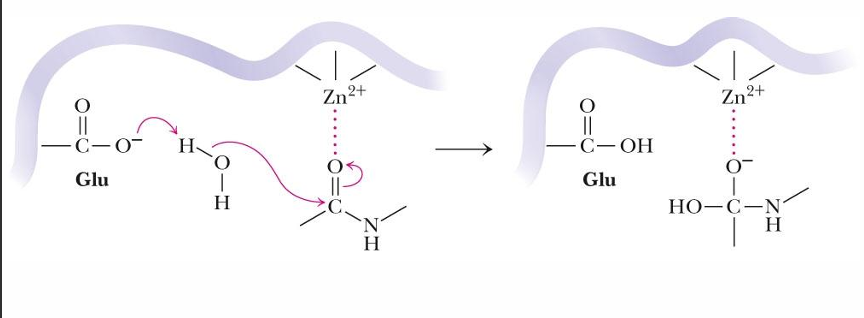
explain what is happening in this reaction
enzyme is using Zn to polarize a carbonyl group (stabilizes neg charge) and Glu to activate water for nuc attack (gen base)
substrate undergoes nuc attack to form intermediate
what happens in covalent catalysis?
substrate is bound covalently to the enzyme to form a reactive intermediate
nucleophilic catalysis is more common
what is an important property of enzymes?
they have the ability to couple reactions
new pathway for lower activation energy
why is the serine protease mechanism important?
understand how chymotrypsin works
cleaves peptide bonds adjacents to aromatic amino acids (Tyr, Trp, Phe)
illustrates the principle of transition-state stabilization
illustrates the use of general acid-base catalysis and covalent catalysis
what is something special about serine proteases?
they can cleave simple organic esters
what does it mean for serine protease to display “burst” kinetics?
rapid release of product followed by a slower, steady rate
suggests that the enzyme quickly forms a covalent intermediate with the substrate
breakdown of that intermediate is slower
first product is released fast while second product is released slow
what are the highlights of the serine protease mechanism?
it is a mixture of covalent and general acid-base catalysis
Asp102 functions only to orient His57
His57 acts as a general acid and base
Ser195 forms a covalent bond with peptide to be cleaved
covalent bond formation turns a trigonal C into a tetrahedral C
tetrahedral oxyanion intermediate is stabilized by amid protons of Gly193 and Ser195
what is the catalytic triad in serine protease?
Asp → stabilizes His
Ser → nuc
His → general acid/base
describe the first step of hydrolytic cleavage of a peptide bond by chymotrypsin
chymotrypsin is the free enzyme
it has an active site (very similar in all the serine proteases)
it has a hydrophobic pocket (interactions specially bind Tyr, Phe, Trp)
substrate binds → side chain of the residue adjacent to the peptide bond to be cleaved goes in a hydrophobic pocket on the enzyme, which positions the PB for attack
Once step 1 occurs, what is formed?
an ES complex
the substrate is in a hydrophobic pocket
the interaction between His57 and Ser195 created a strong nuc alkoxide ion on Ser195
ion attack the peptide carbonyl group (short lived negative charge on the carbonyl oxygen of the substrate, which stabilized by HB in oxyanion hole)
think about the OH donating pair to C=O and the double bond donating to O… HB happens
what is step 2 of Hydrolytic Cleavage of a Peptide Bond by Chymotrypsin?
there is a short-lived intermediate
there is instability of neg charge on the substrate bc of the HB
reformation of double bond with C displaces the bond between amino group of peptide linakge… PEPTIDE BREAKAGE
amino group is protonated by His57
NOT the intermediate with appreciable lifetime
what is step 3 of Hydrolytic Cleavage of a Peptide Bond by Chymotrypsin?
the first product is made: part of the substrate that was pronated by the His57
Leaving an acyl enzyme intermediate
It leaves the intermediate responsible for the steady state velocity
what is step 4 of Hydrolytic Cleavage of a Peptide Bond by Chymotrypsin?
incoming water molecule is deprotonated by general base catalysis → strong nuc hydroxide ion
attack of hydroxide on ester linkage → neg charge in oxyanion hole
what is step 5 of Hydrolytic Cleavage of a Peptide Bond by Chymotrypsin?
collapse to form second product
neg charge goes away by reformation of double bond then O on the other side is LG
displacement of Ser195
what is step 6 of Hydrolytic Cleavage of a Peptide Bond by Chymotrypsin?
the enzyme-product 2 complex!!
what is step 7 of Hydrolytic Cleavage of a Peptide Bond by Chymotrypsin?
the second product is released but its the product from the hydrophobic pocket
leaves what we originally started with
what is interesting about serine proteases?
they all have identical mechanisms
they have different substrate specificity
there is also Trysin: long, positively charges side chains
there is also elastase: small side chains
what is subtilisin?
bacterial serine protease with a diff overall structure
uses the same catalytic triad
it is non specific
no sequence homology with chymotrypsin
what did the mutation analysis of the catalytic triaf show?
each mutant droped down to 10-5s-1
one residue missing disrupts catalysis but some residual activity remains
triad works as a cooperative system
why is the specificity pocket important?
it determines which peptide are cleaved by serine proteases
chymotrypsin → large, hydrophobic for tyr
trypsin → narrow, negatively charged for arg
elastase → small, hydrophobic for ala
what are other classes of proteases?
cysteine proteases
aspartyl proteases
metalloproteases
Describe cysteine proteases
residue activated by histidine residue plays a role of the nuc that attacks the peptide bond
better nuc compared to serine proteases
only has cys and his
happens in papain, cathepsins, and caspases
Describe aspartyl proteases
pair of aspartic acid residues act together to allow a water molecule to attack the peptide bond