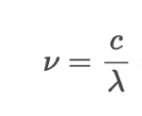SI units conversions
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
Deci
0.1 or 1×10-1 meters (or whatever your base unit is)
Centi
1×10-2 meters (or whatever your base unit is) also 0.01
Milli
1×10-3 meters (or whatever your base unit is) also 0.001
Micro
1×10-6 meters (or whatever your base unit is)
Nano
1×10-9 meters (or whatever your base unit is)
Pico
1×10-12 meters (or whatever your base unit is)
Tera
1×1012 meters (or whatever your base unit is)
Giga
1×109 meters (or whatever your base unit is)
Mega
1×106 meters (or whatever your base unit is)
Kilo
1×103 meters (or whatever your base unit is)
Hecta
1×102 meters (or whatever your base unit is)
Deka
1×101 meters (or whatever your base unit is)
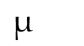
What symbol is this
Micro
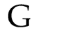
What symbol is this
Giga
What symbol is k?
Kilo
What symbol is p?
Pico
What symbol is n?
Nano
What symbol is M?
Mega
What symbol is T?
Tera
Formal charge equals
(Number of valence electrons on a neutral atom on the periodic table) — (number of valence electrons assigned)
# of valence electrons assigned =
# of electrons in lone pairs + 1/2(# of electrons in bonds)
What is the electron pair geometry of a molecule with 2 electron domains?
Linear
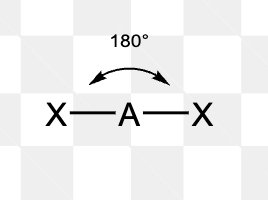
What is the electron pair geometry of a molecule with 3 electron domains?
Trigonal planar
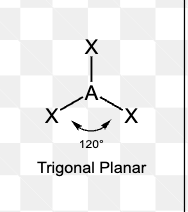
What is the electron pair geometry of a molecule with 4 electron domains?
Tetrahedral
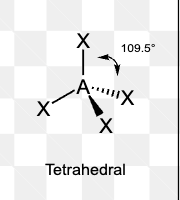
What is the electron pair geometry of a molecule with 5 electron domains?
Trigonal bipyramidal
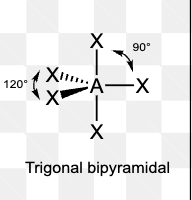
What is the electron pair geometry of a molecule with 6 electron domains?
Octahedral
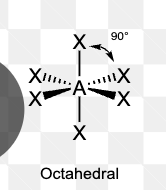
What is the molecular geometry of a molecule with 3 electron domains and 1 lone pair?
Bent
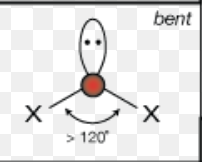
What is the molecular geometry of a molecule with 4 electron domains and 1 lone pair?
trigonal pyramidal
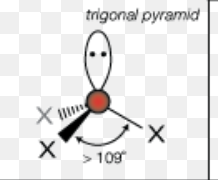
What is the molecular geometry of a molecule with 4 electron domains and 2 lone pairs?
bent (v-shaped)
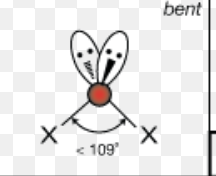
What is the molecular geometry of a molecule with 5 electron domains and 2 lone pairs?
T-shaped
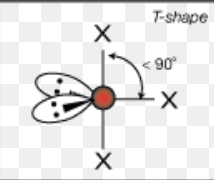
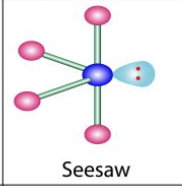
What is the molecular geometry of a molecule with 5 electron domains and 1 lone pair?
Seesaw
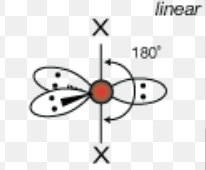
What is the molecular geometry of a molecule with 5 electron domains and 3 lone pairs?
Linear
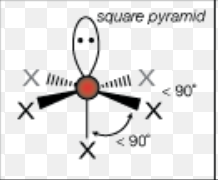
What is the molecular geometry of a molecule with 6 electron domains and 1 lone pairs?
square pyramidal
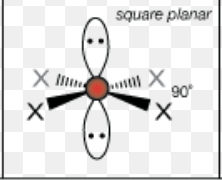
What is the molecular geometry of a molecule with 6 electron domains and 2 lone pairs?
square planar
Equation to calculate energy of a photon/ change in energy between energy transitions
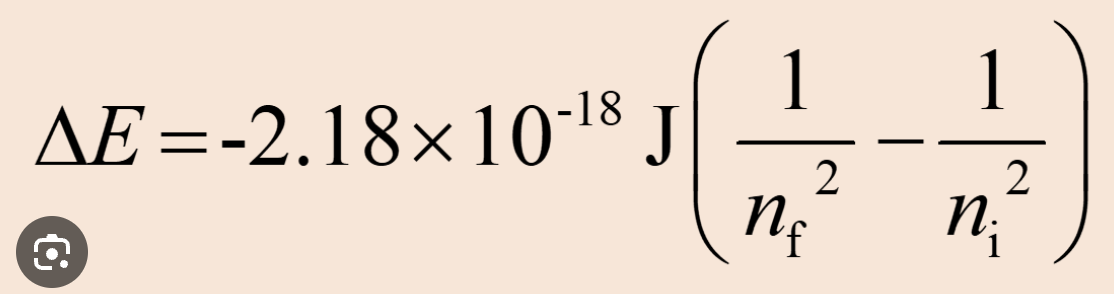
Equation to calculate the wavelength from the change in energy
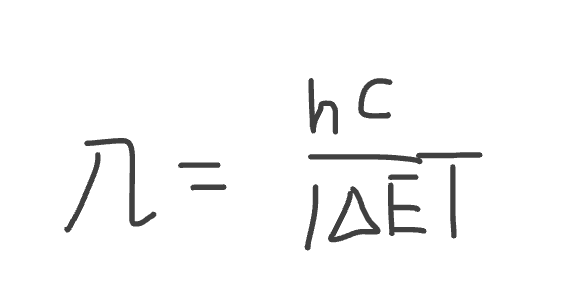
Equation to calculate v from energy
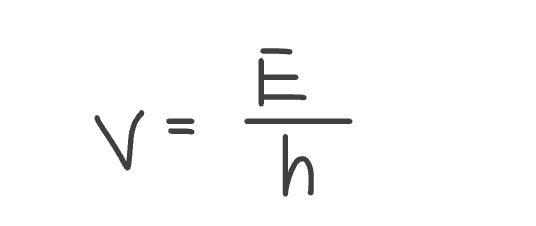
Equation to calculate wavelength using h and c
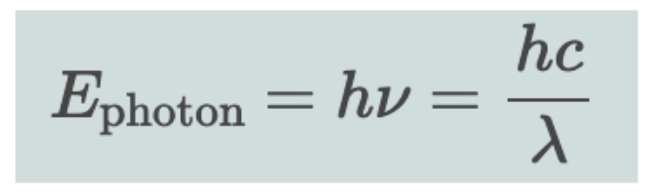
Equation to calculate v from wavelength