A&P exam 1
1/202
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
203 Terms
organization
levels of biological organization extends within (micro) and beyond (macro) the individual as follows:
atoms --> molecules --> organelles --> cells --> tissues --> organs --> organ systems --> organisms --> populations --> communities --> ecosystems --> biosphere
matter
has mass and occupies space, three forms are solid (bone), liquid (blood), and gas (oxygen)
atom
smallest particle exhibiting chemical properties of an element, 92 naturally occurring elements make up matter, organized in periodic table of elements
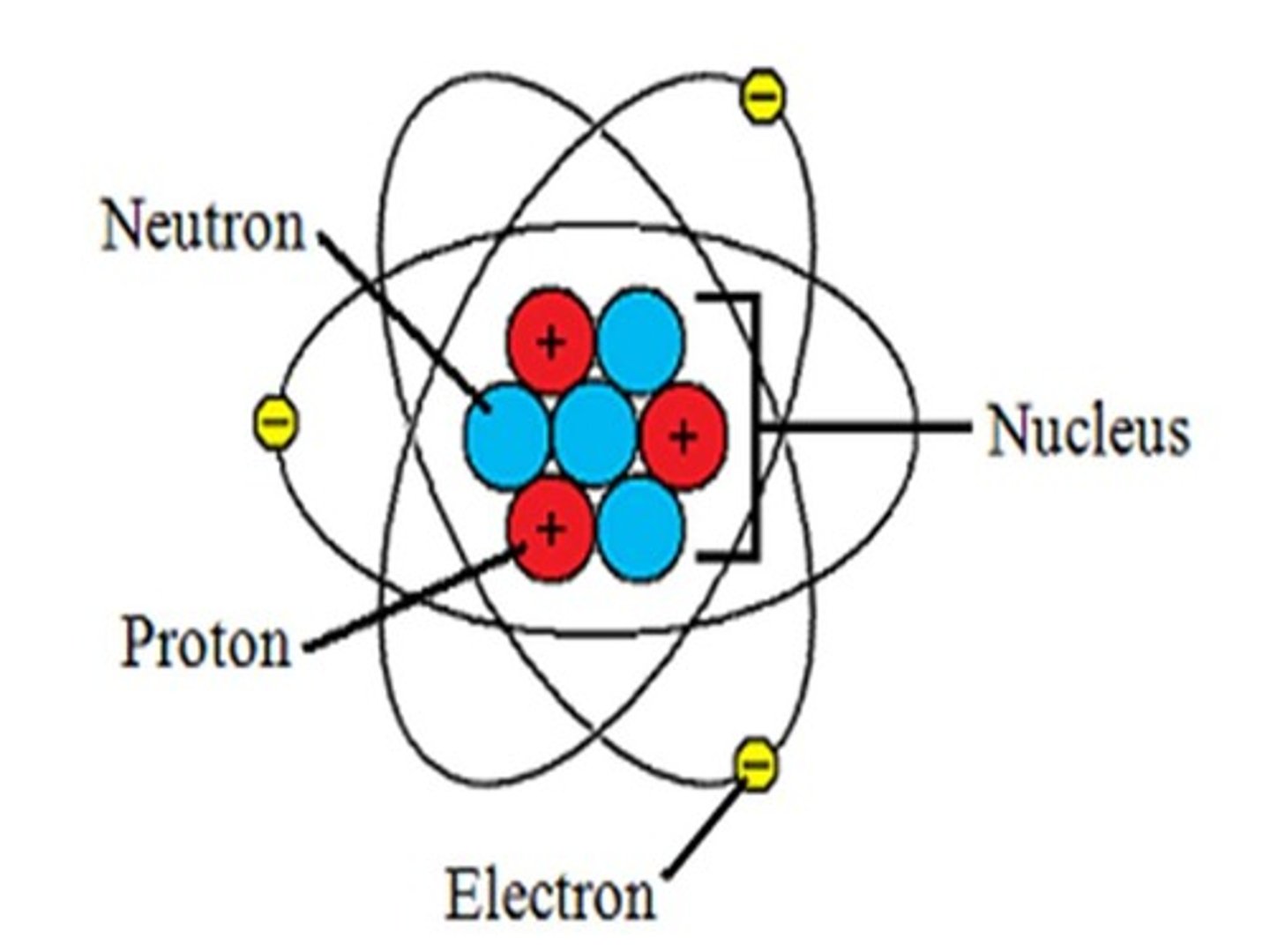
components of atom
composed of three subatomic particles: neutrons, protons, electrons
neutrons
mass of one atomic mass unit (amu), no charge
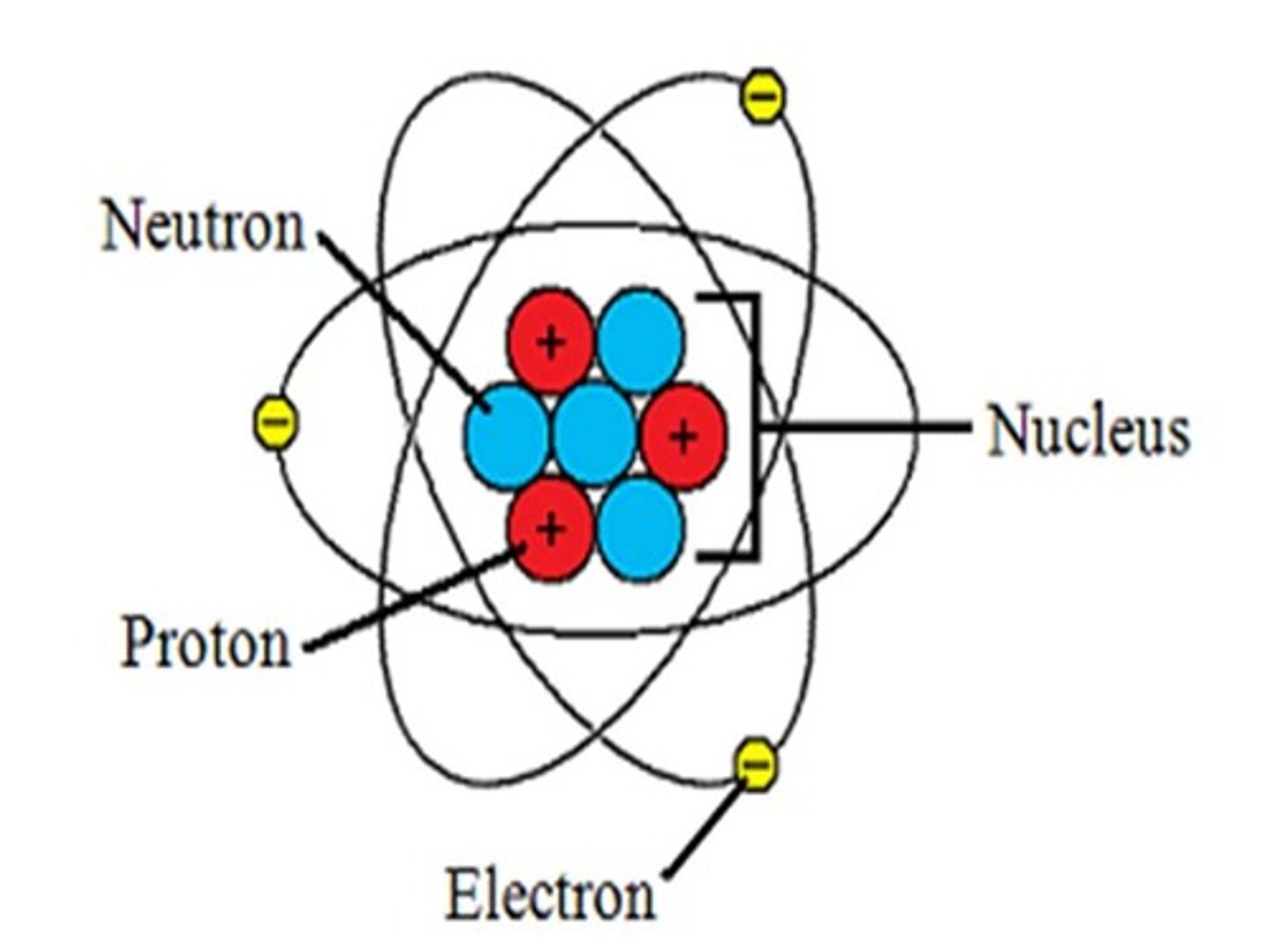
protons
mass of one amu, positive charge of one (+1)
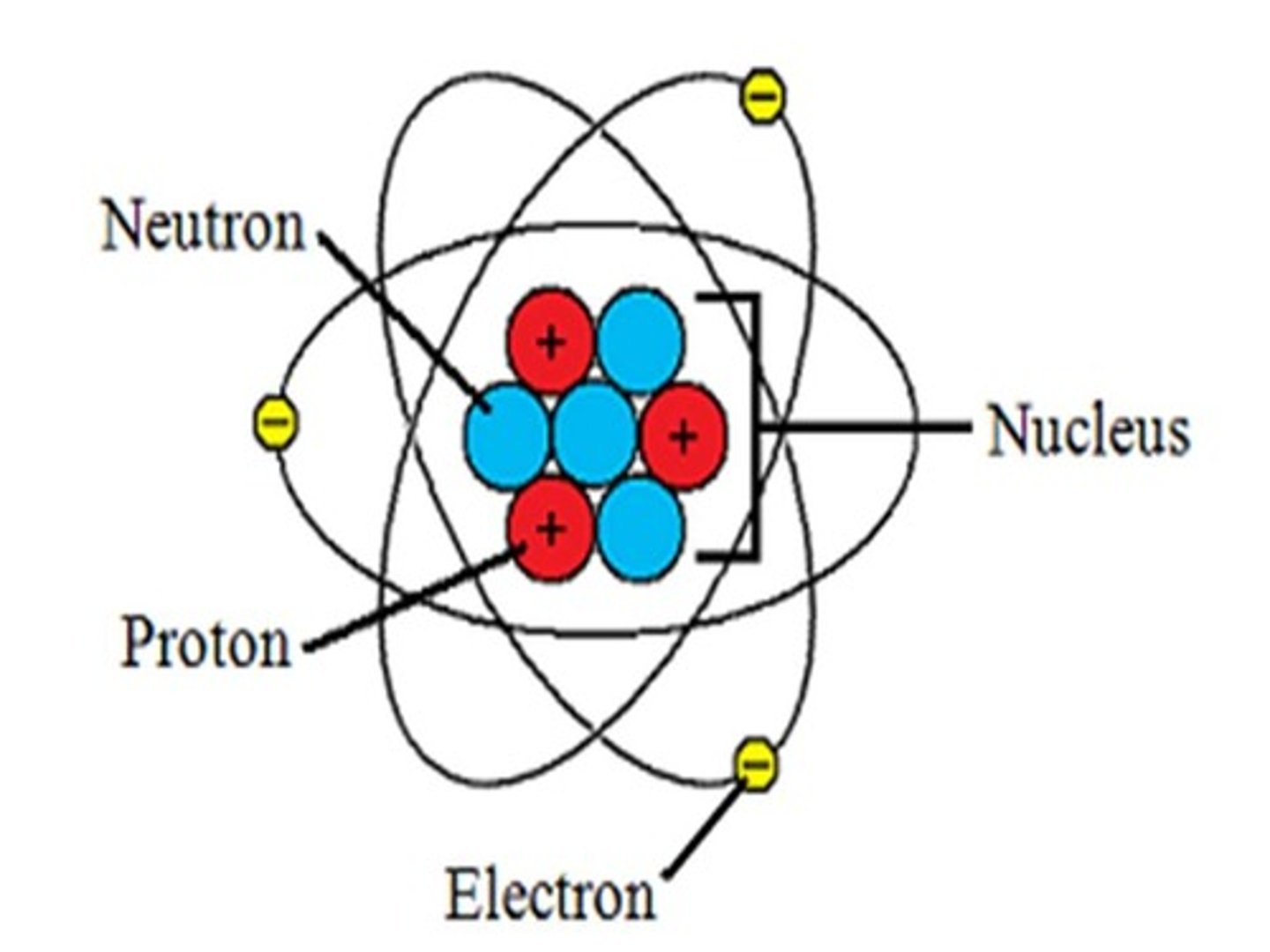
electrons
negative charge of one (-1), located at varying distance from nucleus in regions called orbitals
chemical symbol of periodic table
unique to each element, usually identified by first letter or first letter plus an additional letter
atomic number of periodic table
number of protons in atom of element, located above symbol name, elements arranged by anatomic number within rows
average atomic mass of periodic table
mass of both protons and neutrons, shown below element's symbol on table
determining the number of subatomic particles
proton number = atomic number
neutron number = atomic mass - atomic number ((p + n) - p)
electron number = proton number
diagramming atomic structures
atom has shells of electrons surrounding nucleus, each shell has given energy level, each shell holds limited number of electrons, innermost shell: two electrons, second shell up to eight, shells close to nucleus must be filled first
isotope
different atoms of same element, same number of protons and electrons, different number of neutrons, identical chemical characteristics, different atomic masses
i.e. carbon-12 with 6 neutrons, carbon-13 with 7 neutrons, carbon-14 with 8 neutrons
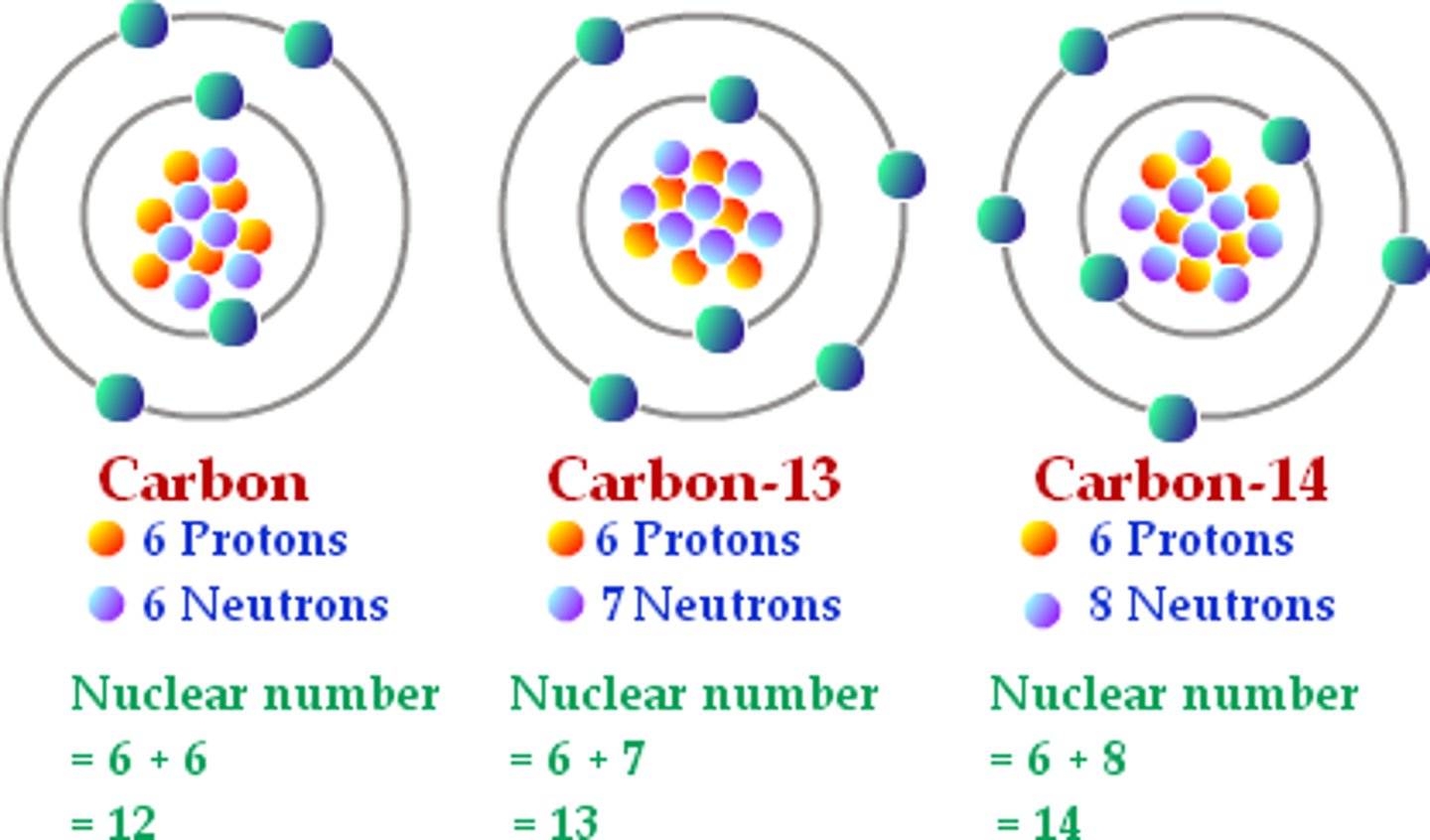
average atomic mass
weighted average of atomic mass for all isotopes
radioisotopes
contain excess neutrons, so unstable, lose nuclear components in form of high energy radiation
i.e. alpha particles, beta particles, gamma ray
physical half-life
time for 50% of radioisotope to become stable, can vary from a few hours to thousands of years
biological half-life
time required for half of radioactive material from test to be eliminated from body
chemical compounds
stable associations between two or more elements combined in fixed ratio, classified as ionic or molecular
ionic compounds
structures composed of ions held together in lattice by ionic bonds
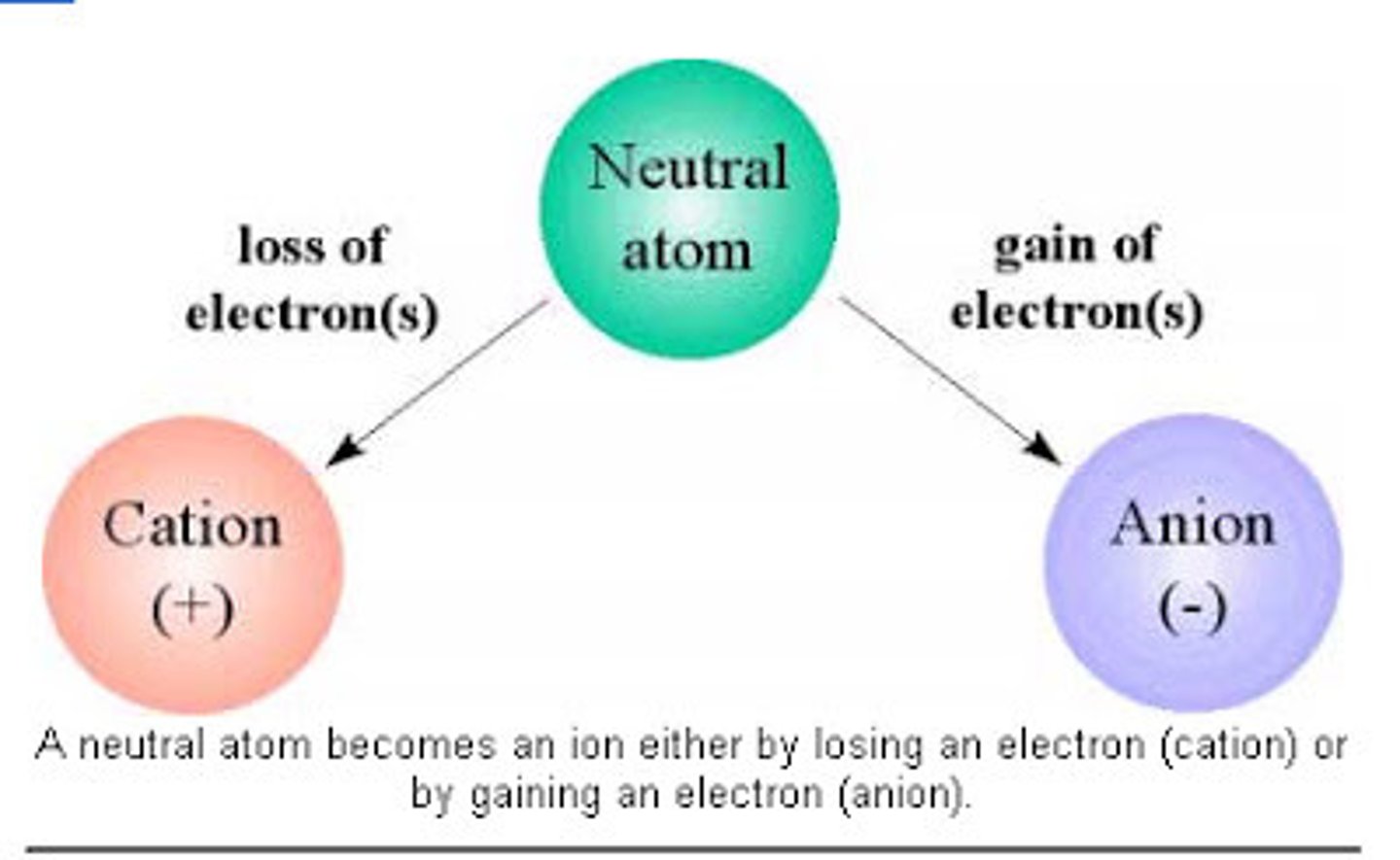
ions
atoms with positive or negative charge, produced from loss or gain of one or more electrons, significant physiological functions (K+ is used in sports drinks to replace K+ lost in sweat, K+ in large dose is used in some states for lethal injection)
cations
ions with positive charge
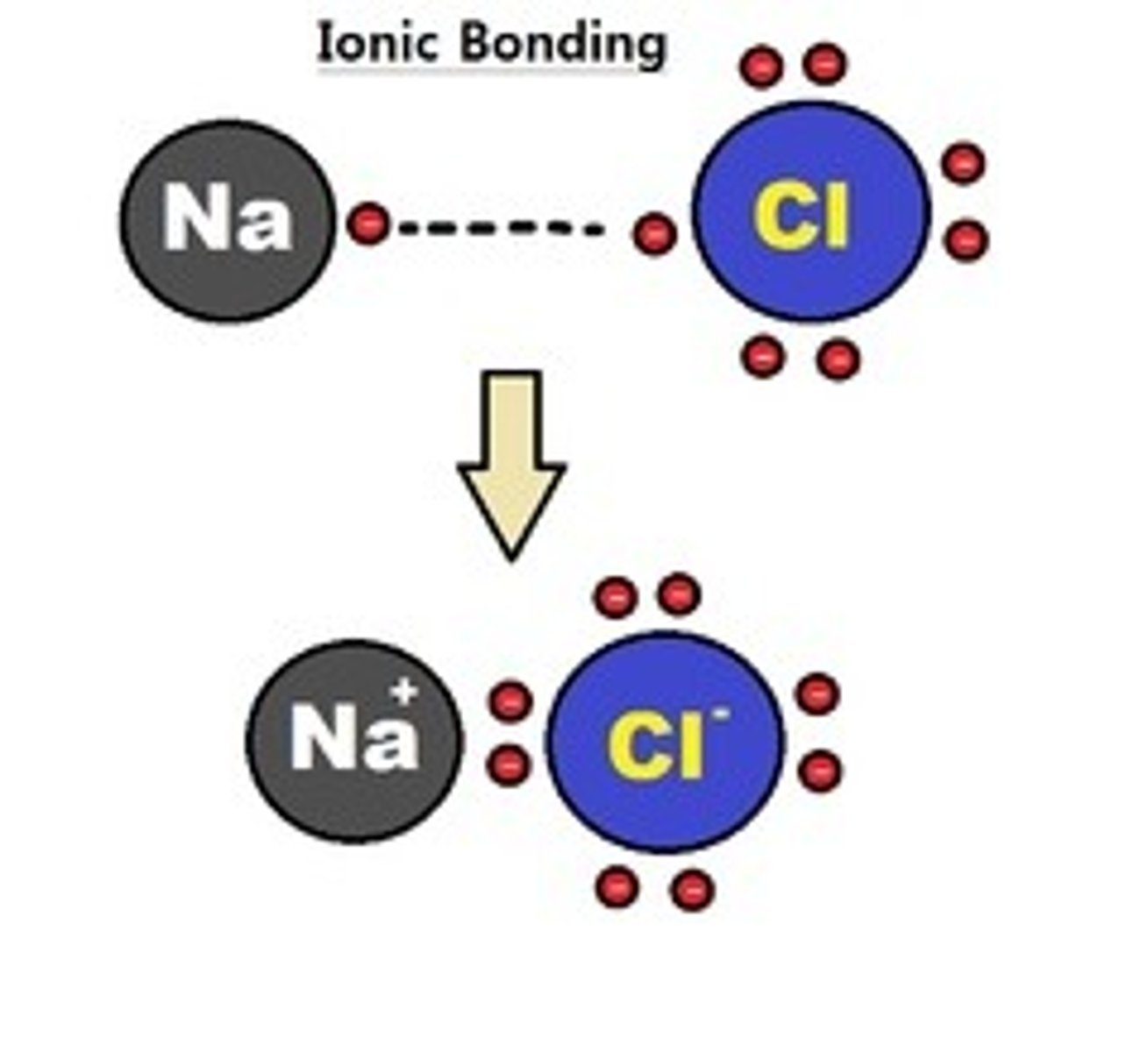
sodium can reach stability by donating electron, now satisfies octet rule, now has 11 protons and 10 electrons, charge is +1
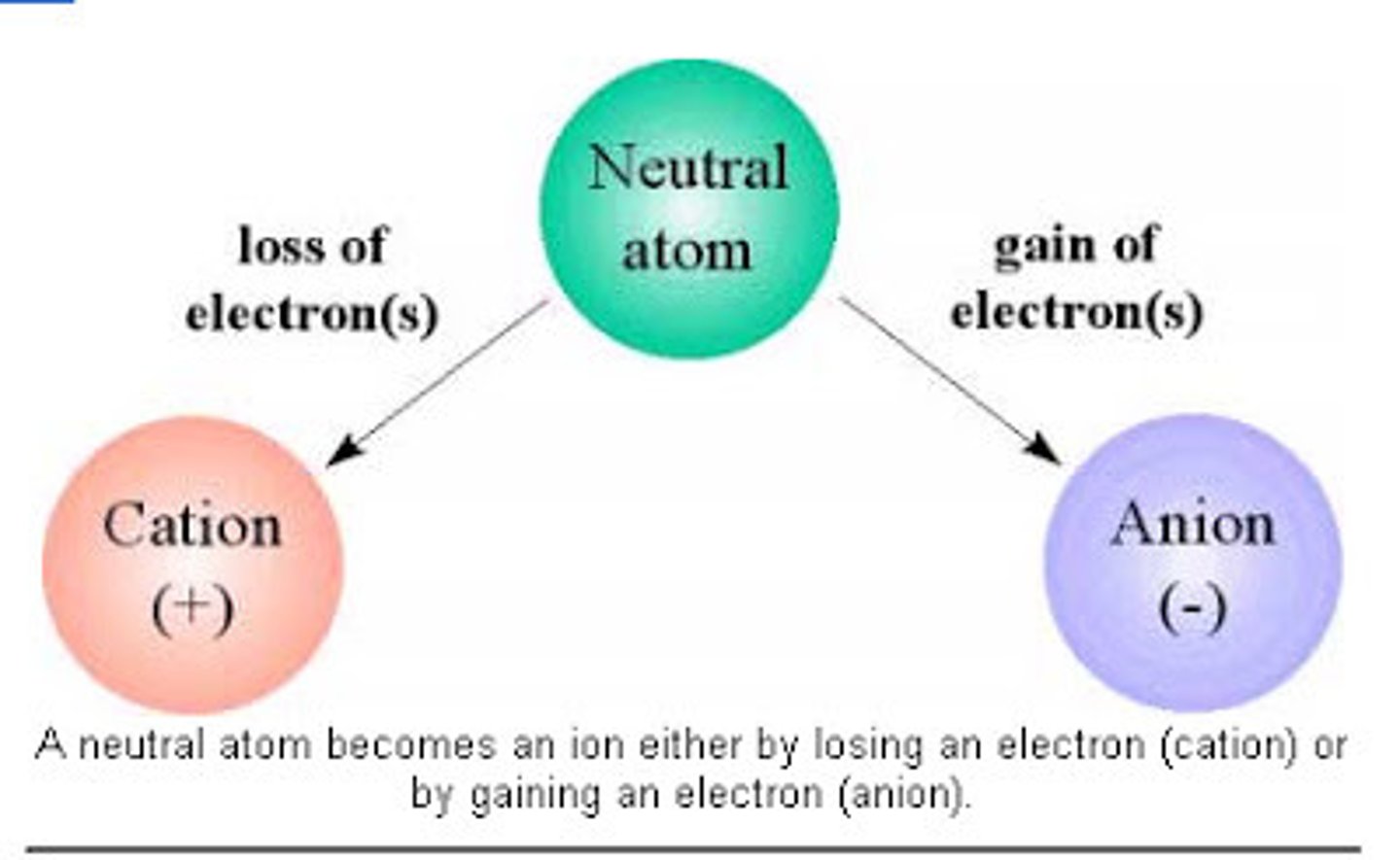
anions
ions with negative charge
chlorine reaches stability by gaining electron, now satisfies octet rule, now has 17 protons and 18 electrons, charge is -1
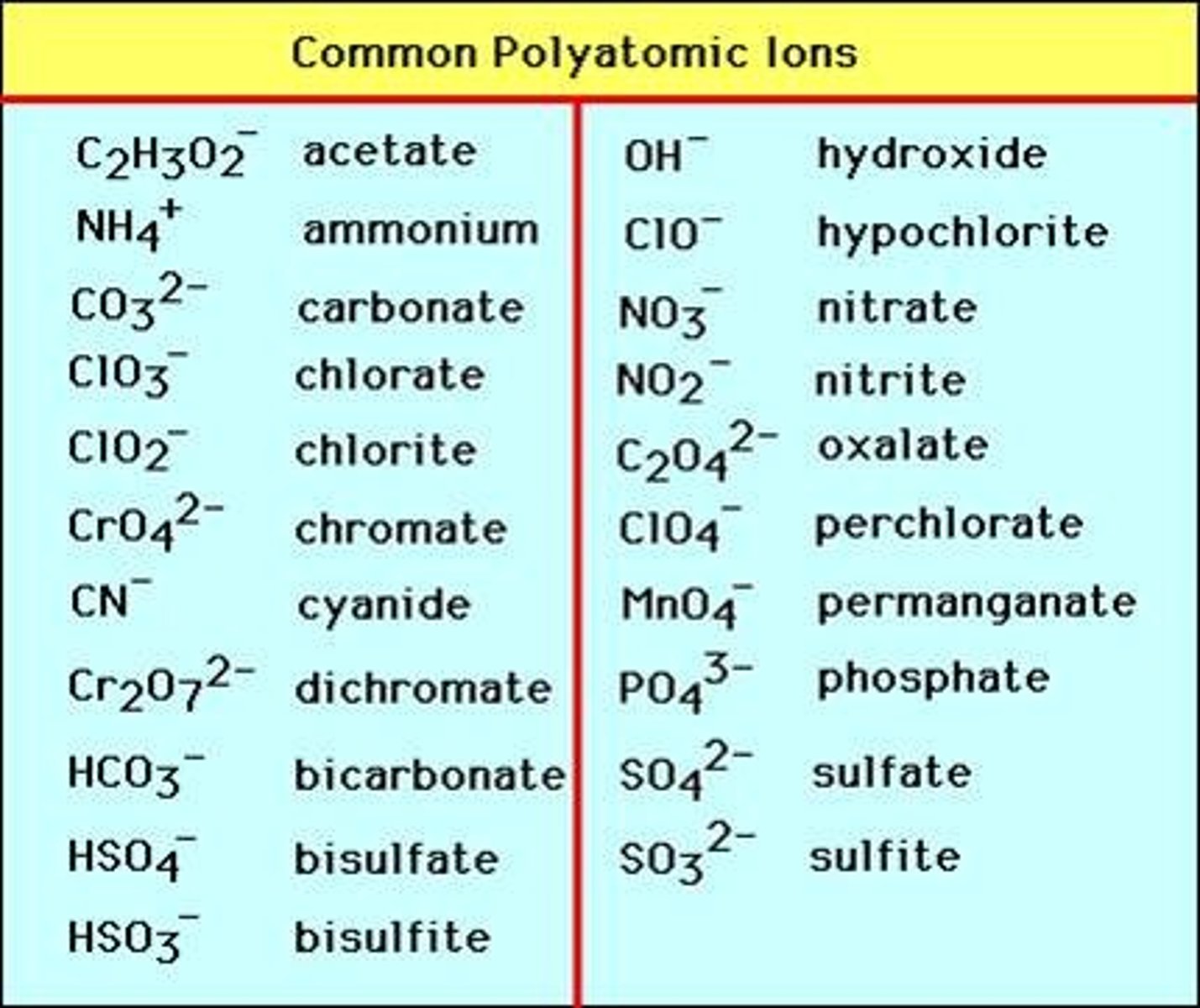
polyatomic ions
anions with more than one atom
i.e. bicarbonate ion and phosphate ion
ionic bonds
cations and anions bound by electrostatic forces, form salts
i.e. table salt (NaCl), each sodium atom loses one outer shell electron to chlorine atom, sodium and chlorine ions are held together by ionic bonds in lattice crystal structure (ionic compound)
i.e. magnesium chloride, each magnesium atom loses one electron to each of the two chlorine atoms
covalently bonded molecule
electrons shared between atoms of two or more different elements, termed molecular compounds
i.e. carbon dioxide (CO2), but not molecular oxygen (O2) (bc only one atom)
occurs when both atoms require electrons, occurs with atoms with 4 to 7 electrons in outer shell, formed commonly in human body using
i.e. H, O, N, C
simplest occurs between two hydrogen atoms - each sharing its single electron, oxygen needs two electrons to complete outer shell - forms two covalent bonds, nitrogen forms three bonds, carbon forms four bonds
molecular formula
indicates number and type of atoms
i.e. carbonic acid (H2CO3)
structural formula
indicated number and type of atoms, indicated arrangement of atoms within molecule, allows differentation of isomers, same number and type of elements but arranged differently in space
i..e O = C = O (carbon dioxide)
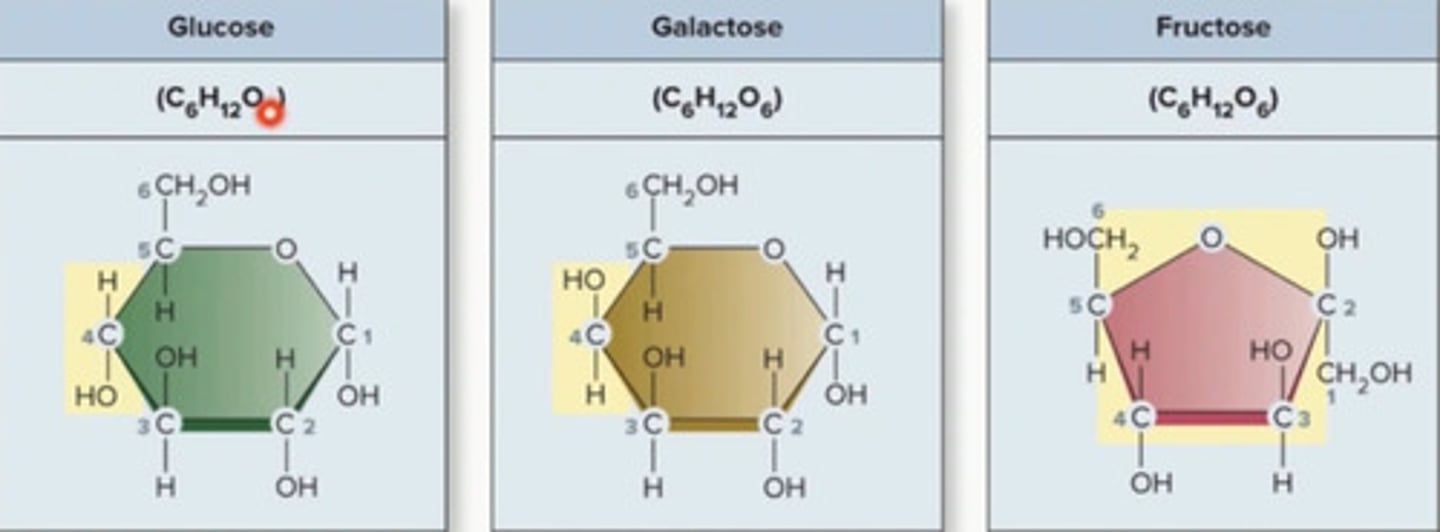
glucose vs galactose vs fructose
same molecular formula, 6 carbon 12 hydrogen 6 oxygen, atoms arranged differently, isomers may have different chemical properties
single covalent bond
one pair of electrons shared, i.e. between two hydrogen atoms
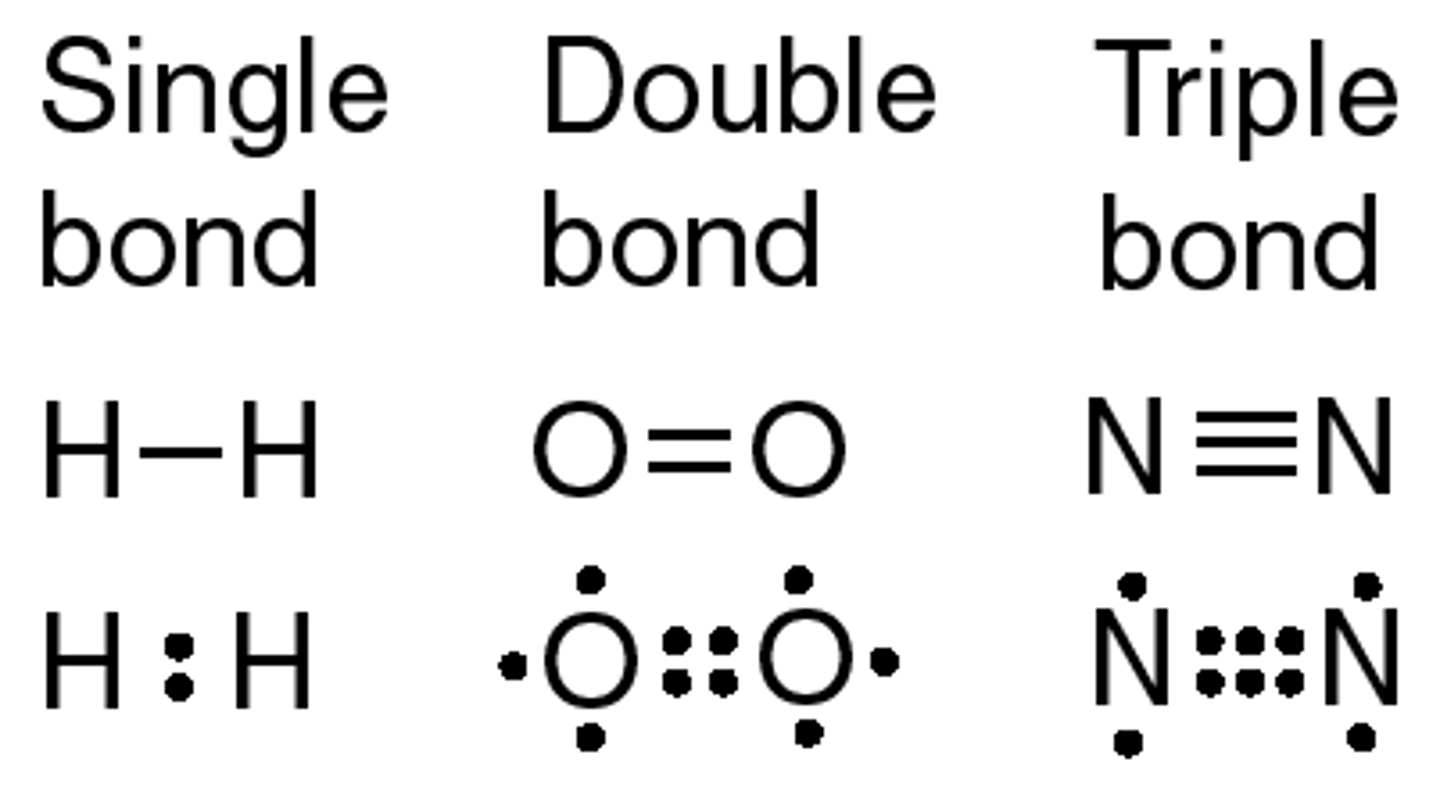
double covalent bond
two pairs of electrons shared, i.e. between two oxygen atoms
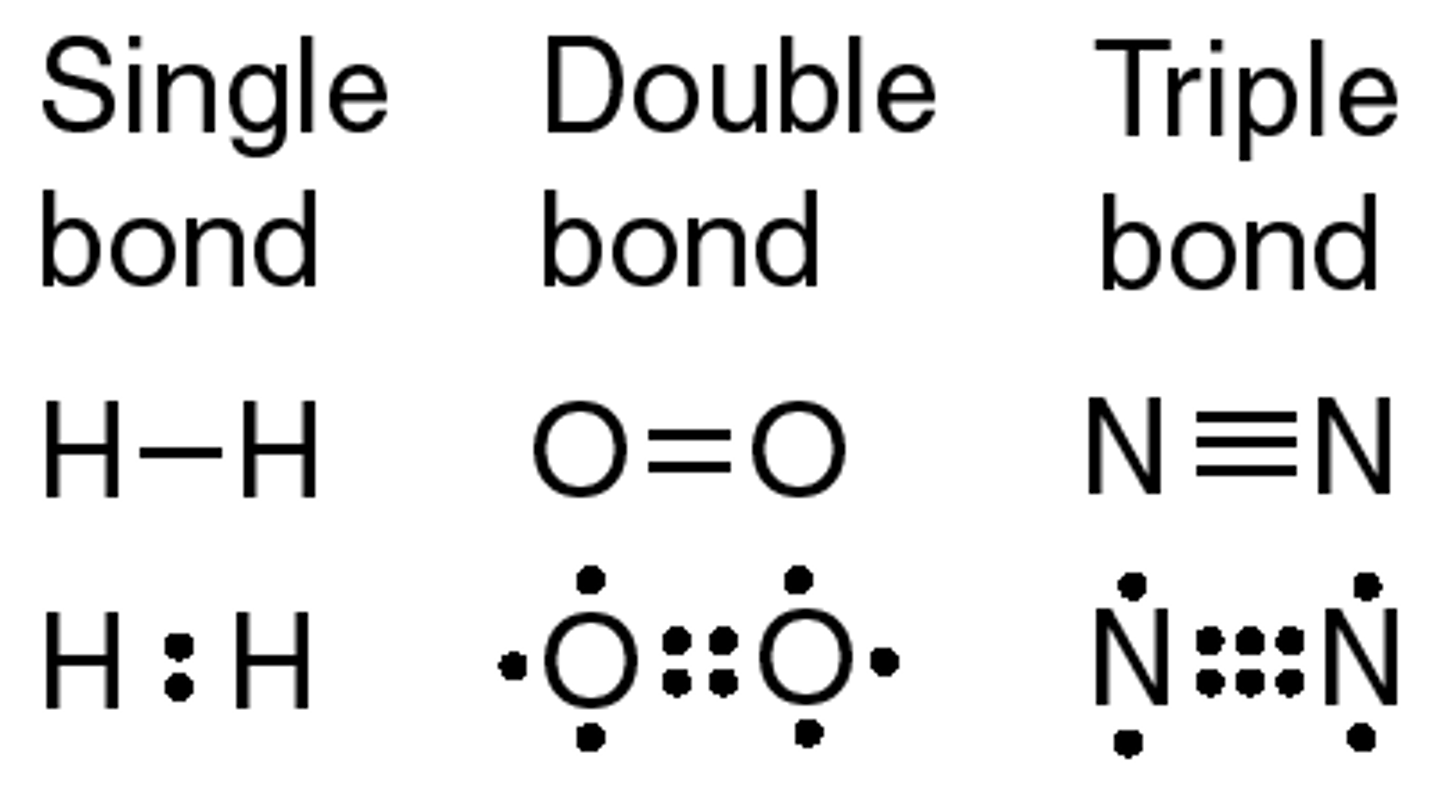
triple covalent bond
three pairs of electrons shared, i.e. between two nitrogen atoms
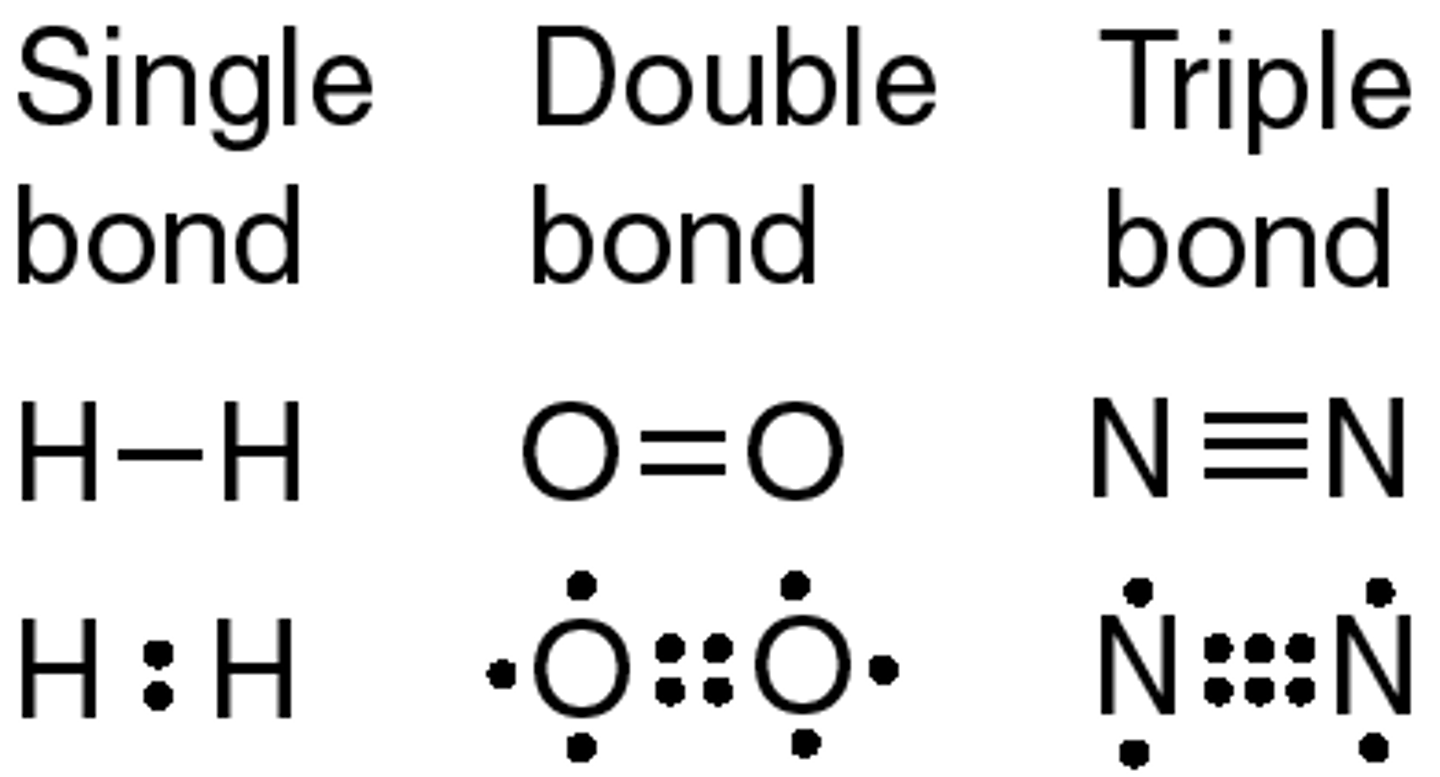
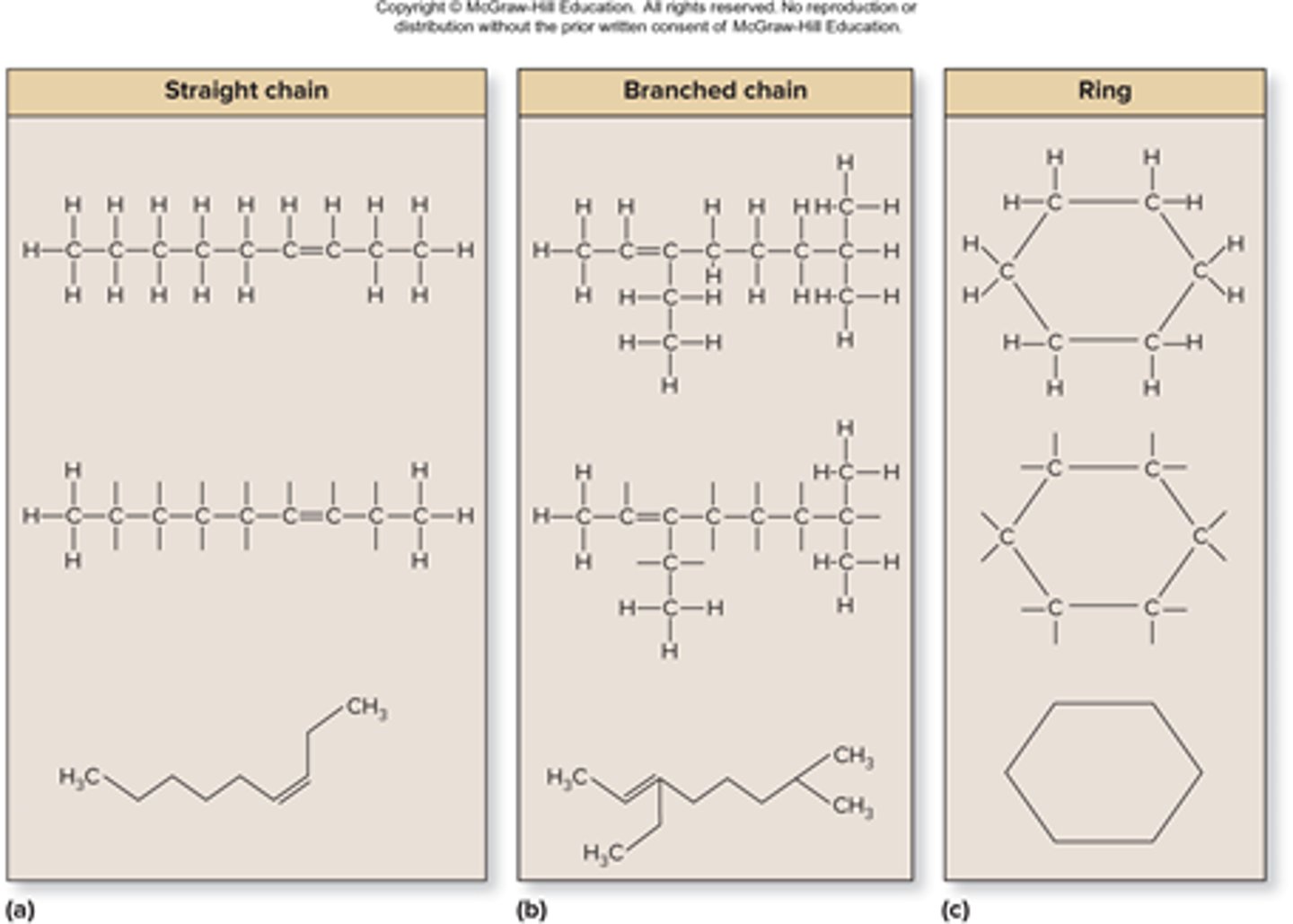
carbon skeleton formation
bonds in straight chains, branched chains, or rings, carbon present where lines meet at angle, additional atoms are hydrogens
electronegativity
relative attraction of each atom for electrons, determines how electrons are shared in covalent bonds, in periodic table electronegativity increases from left to right across row and from bottom to top in column
i.e. hydrogen < carbon < nitrogen < oxygen
more electronegative atom develops partial negative charge (δ−), less electronegative atoms develops partial positive charge (δ+), exception to rule of polar bond forming between two different atoms (carbon bonding with hydrogen)
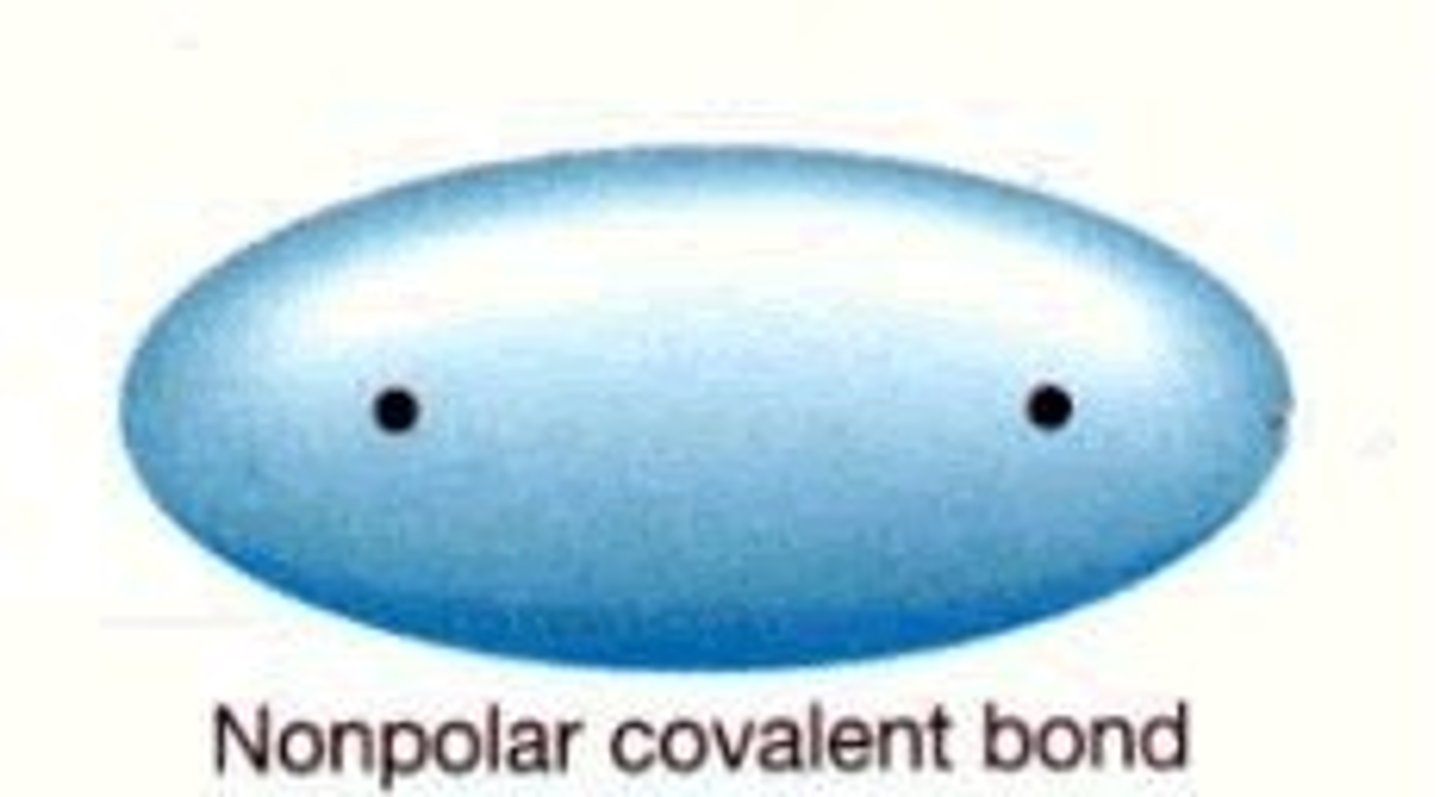
nonpolar covalent bond
two atoms of same element have equal attraction for electrons, nonpolar molecules contain nonpolar covalent bonds, nonpolar molecules may contain polar covalent bonds if polar covalent bonds cancel each other (i.e. carbon dioxide)
i.e. O - O and C - H are nonpolar bonds
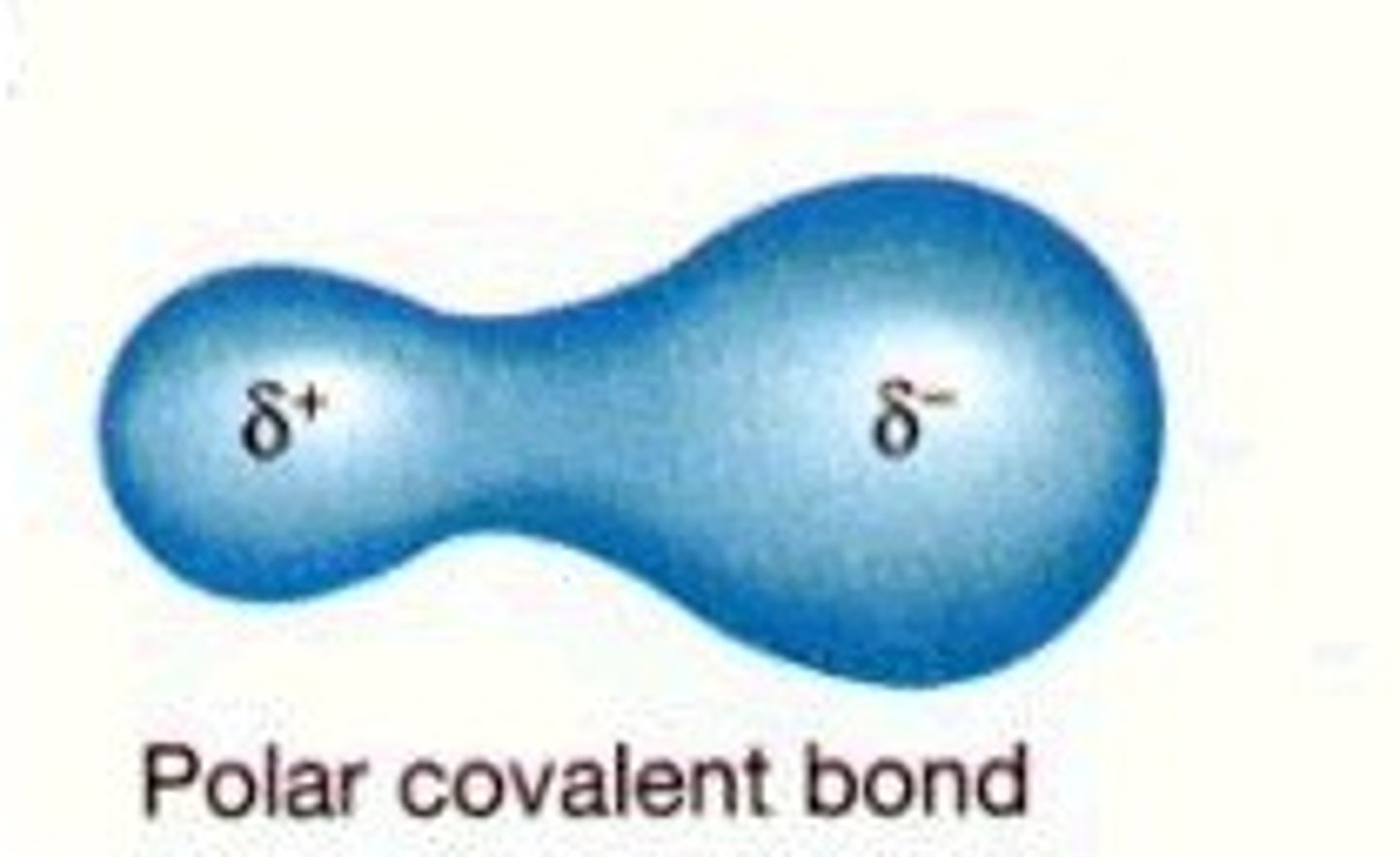
polar covalent bond
sharing of electrons unequally, polar molecules contain polar covalent bonds
i.e. O - H is polar bond in polar molecule water (H2O)
amphipathic molecules
large molecules with both polar and nonpolar regions, i.e. phospholipids
intermolecular attractions
weak chemical attractions between molecules, important for shape of complex molecules
i.e. DNA and proteins
hydrogen bond
forms between polar molecules, attraction between partially positive hydrogen atom and partially negative atom, individually weak, collectively strong, influences how water molecules behave
van der waals forces
nonpolar molecules, electrons orbiting nucleus briefly unevenly distributed, induce unequal distribution of adjacent atom of another nonpolar molecule, individually weak
hydrophobic interaction
excluded molecules, nonpolar molecules placed in polar substance, if occurring between parts of large molecules then termed intramolecular attractions
molecular structure of water
composes 2/3 of human body by weight, polar molecule, one oxygen atom bonded to two hydrogen atoms, oxygen atom has two partial negative charges, hydrogens have single partial positive charge, can form four hydrogen bonds with adjacent molecules, central to water's properties
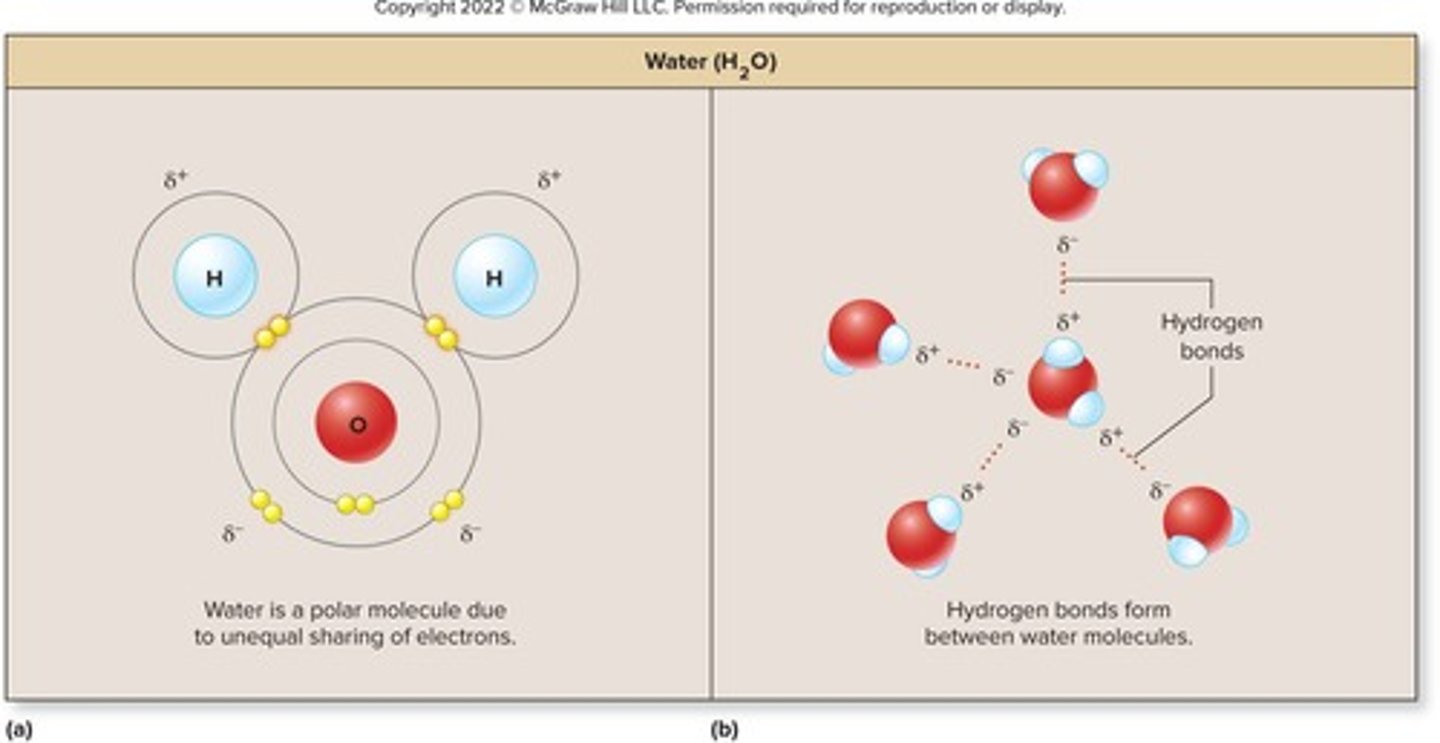
three phases of water (depending on temperature)
(1) gas (water vapor), substances with low molecular mass (2) liquid (water), almost all water in the body, liquid at room temperature due to hydrogen bonding (3) solid (ice)
functions of liquid water
transports: substances dissolved in water move easily throughout body, lubricates: decreases friction between body structures, cushion: absorbs sudden force of body movements, excretes wastes: unwanted substances dissolve in water are easily eliminated
properties of water
cohesion, surface tension, adhesion, temperature, specific heat, heat of vaporization,
cohesion
attraction between water molecules due to hydrogen bonding
surface tension
inward pulling of cohesive forces at surface of water, causes moist sacs of air in lungs to collapse, surfactant, a lipoprotein, prevents collapse
adhesion
attraction between water molecules and substance other than water
temperature
measure of kinetic energy of atoms or molecules within substance
i.e. vasoconstriction and vasodilation
specific heat
amount of energy required to increase temperature of 1 gram of substance by 1 degree Celsius, water's value extremely high due to energy needed to break hydrogen bonds, contributes to keeping body temperature constant
heat of vaporization
heat required for release of molecules from a liquid phase into a gaseous phase for 1 gram of a substance, water's value very high due to hydrogen bonding, sweating cools body, excess heat dissipated as water evaporates
substances that dissolve in water
polar molecules and ions (hydrophilic), water surrounds substances and forms hydration shell, (1) some substances dissolve but remain intact (glucose and alcohol), nonelectrolytes remain intact but do not conduct current, (2) substance dissolve and dissociate (separate), NaCl dissolves into Na+ and Cl- ions, acids and bases (HCl), electrolytes can conduct current
substances that do not dissolve in water
nonpolar molecules (hydrophobic), hydrophobic substances require carrier proteins to be transported within blood
i.e. fats and cholesterol are unable to dissolve within water
hydrophobic exclusion
cohesive water molecules "force out" nonpolar molecules
substances that partially dissolve in water
amphipathic molecules have polar and nonpolar regions, polar portion of molecule dissolves in water, nonpolar portion repelled by water, phospholipid molecules are amphipathic, polar heads have contact with water, nonpolar tails group together, results in bilayers of phospholipid molecules (membranes of cell), other amphipathic molecules form a micelle
water: a neutral solvent
water spontaneously dissociates to form ions, bond between oxygen and hydrogen breaks apart spontaneously, OH group hydroxide ion (OH-), hydrogen ion transferred to second water molecule (hydronium ion: H3O+), equal numbers of positive hydrogen ions and negative hydroxyl ions produced, water remains neutral
H2O + H2O --> H3O+ + OH- (or simplified: H2O --> H+ + OH-)
acids
dissociate in water to produce H+ and anion, proton donor, increases concentration of free H+, more dissociation of H+ with stronger acids (HCl in stomach), less dissociation of H+ with weaker acids (carbonic acid in blood)
substance A (acid in water) --> H+ + anion
bases
accepts H+ when added to solution, proton acceptor, decreases concentration of free H+, more absorption of H+ with stronger bases (ammonia and bleach), less absorption of H+ with weaker bases (bicarbonate in blood and in secretions released into small intestine)
substance B (base in water) + H+ --> B - H
neutralization
when acidic or basic solution is returned to neutral (pH 7), acids neutralized by adding base (medications to neutralize stomach acid must contain base), bases neutralized by adding acid
buffers
help prevent pH changes if excess acid or base is added, act to accept H+ from excess acid or donate H+ to neutralize base
i.e. carbonic acid (weak acid) and bicarbonate (weak base) buffer blood pH, both help maintain blood pH in critical range (7.35 to 7.45)
mixtures
formed from combining two or more substances, two defining features: substances mixed are not chemically changed, substances can be separated by physical means (evaporation or filtering)
three categories of water mixtures
suspension, colloid, solution
suspension
material larger in size than 1 mm mixed with water, does not remain mixed unless in motion, appears cloudy or opaque, scatters light
i.e. blood cells within plasma or sand in water
emulsion
special category of suspension, water and nonpolar liquid substance, does not mix unless shaken
i.e. oil and vinegar salad dressing or breast milk
colloid
smaller particles than suspension but larger than those in solution, remains mixed when not in motion, scatter light
i.e. fluid in cell cytosol and fluid in blood plasma
solution
homogeneous mixture of material smaller than 1 nm, dissolves in water, does not scatter light, does not settle if solution not in motion
i.e. sugar water, salt water, blood plasma
mass/volume
mass of solute per volume of solution
i.e. results from blood test
mass/volume percent
grams of solute per 100 mL solution
i.e. IV solutions
molarity
moles solute/L solution, alters with changes in temperature, more easily measure in body than molality
molality
moles solute/kg solvent, does not alter with changes in temperature, slightly more accurate than molarity
mole
6.022 x 10^23 atoms, ions, or molecules, mass in grams equal to atomic mass of element or molecular mass of compound
ATP
adenosine triphosphate, nucleotide composed of nitrogenous base adenine, ribose sugar, and three phosphate groups, central molecule in transfer of chemical energy within cell, covalent bonds between last two phosphate groups are unique and energy rich, release energy when broken
important nucleotide-containing molecules
nicotinamide adenine dinucleotide, flavin adenine dinucleotide, both participate in production of ATP
anatomy
studies form and structure of body
physiology
examines how body functions, examine function of body structures, focusing on molecular and cellular level
homeostasis
maintenance of relatively stable internal conditions despite continuous changes in environment, dynamic state of equilibrium, always readjusting as needed, maintained by contributions of all organ systems
hippocrates and aristole
founder of anatomy - herophilus of chalcedon
hippocrates
greek physician, father of western medicine, first to separate disease from superstition, key players in early anatomy
herophilus and eristratus
vivisections of criminals
middle ages, renaissance, 17 and 18th centuries
middle ages: study of anatomy outlawed
renaissance: anatomical interest/knowledge reestablished
17 and 18th centuries: anatomists like celebrities, people paid to see dissections in large amphitheaters
gross or macroscopic anatomy
study of large visible structures, regional anatomy, systemic anatomy (cardiovascular, nervous, muscular), surface anatomy, deep anatomy, comparative anatomy
microscopic anatomy
cytology: microscopic study of cells
histology: microscopic study of tissues
physiology subdisciplines
cardiovascular physiology, neurophysiology, respiratory physiology, reproductive physiology, pathophysiology
cardiovascular physiology
examines functioning of heart, blood vessels, and blood
neurophysiology
studies functioning of nerves and nervous system organs
respiratory physiology
explores functioning of respiratory organs
reproductive physiology
investigates functioning of reproductive hormones and reproductive cycle
pathophysiology
focuses on function of body system during disease or injury to system
anatomic position
upright stance, feet parallel and flat on floor, upper limbs at sides of body, palms face anteriorly (toward front), head is level, eyes look forward
section
slice that exposes internal anatomy
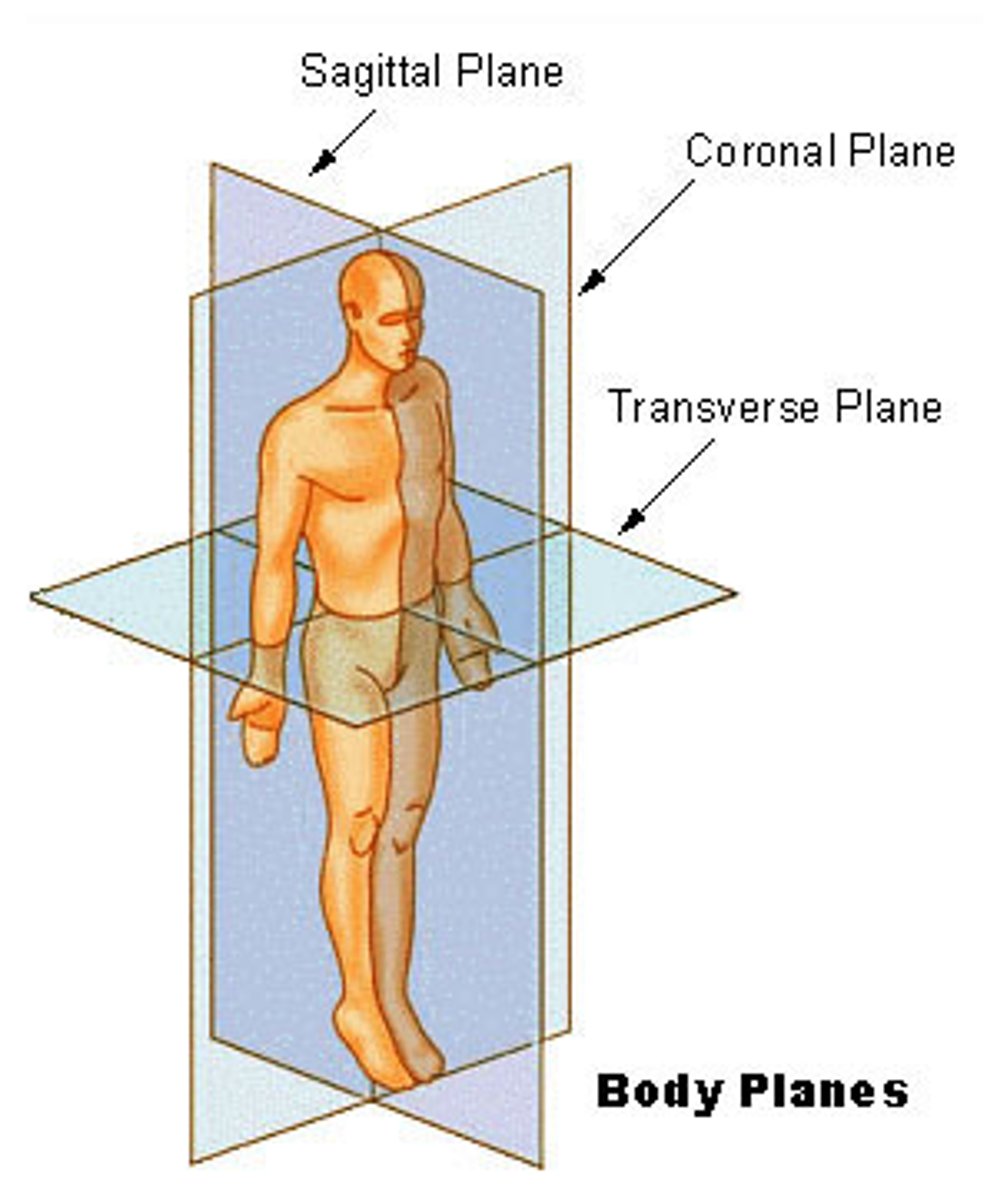
plane
imaginary flat surface passing through body
coronal (or frontal) plane, transverse (or cross-sectional) plane, midsagittal (or median) plane, sagittal plane, oblique plane
anterior/posterior
anterior: in front of, aka ventral, i.e. sternum is anterior to spine
posterior: back of body, behind, aka dorsal, i.e. heart is posterior to ribcage
superior/inferior
medial/lateral
medial: toward middle of body, inner side of, i.e. nose is medial to eye
lateral: away from midline, on other side of, i.e. ear is lateral to eye
proximal/distal
proximal: closer to point of attachment of limb to body trunk, i.e. elbow is proximal to wrist
distal: further from point of attachment of limb to body trunk, i.e. knee is distal to thigh
superficial/deep
superficial: toward body's surface, aka eternal, i.e. skeletal muscles are superficial to bones
deep: away from body's surface, internal, i.e. lungs are deep to ribs
integumentary system
protects body, receives sensory input, helps control temperature, synthesizes vitamin D
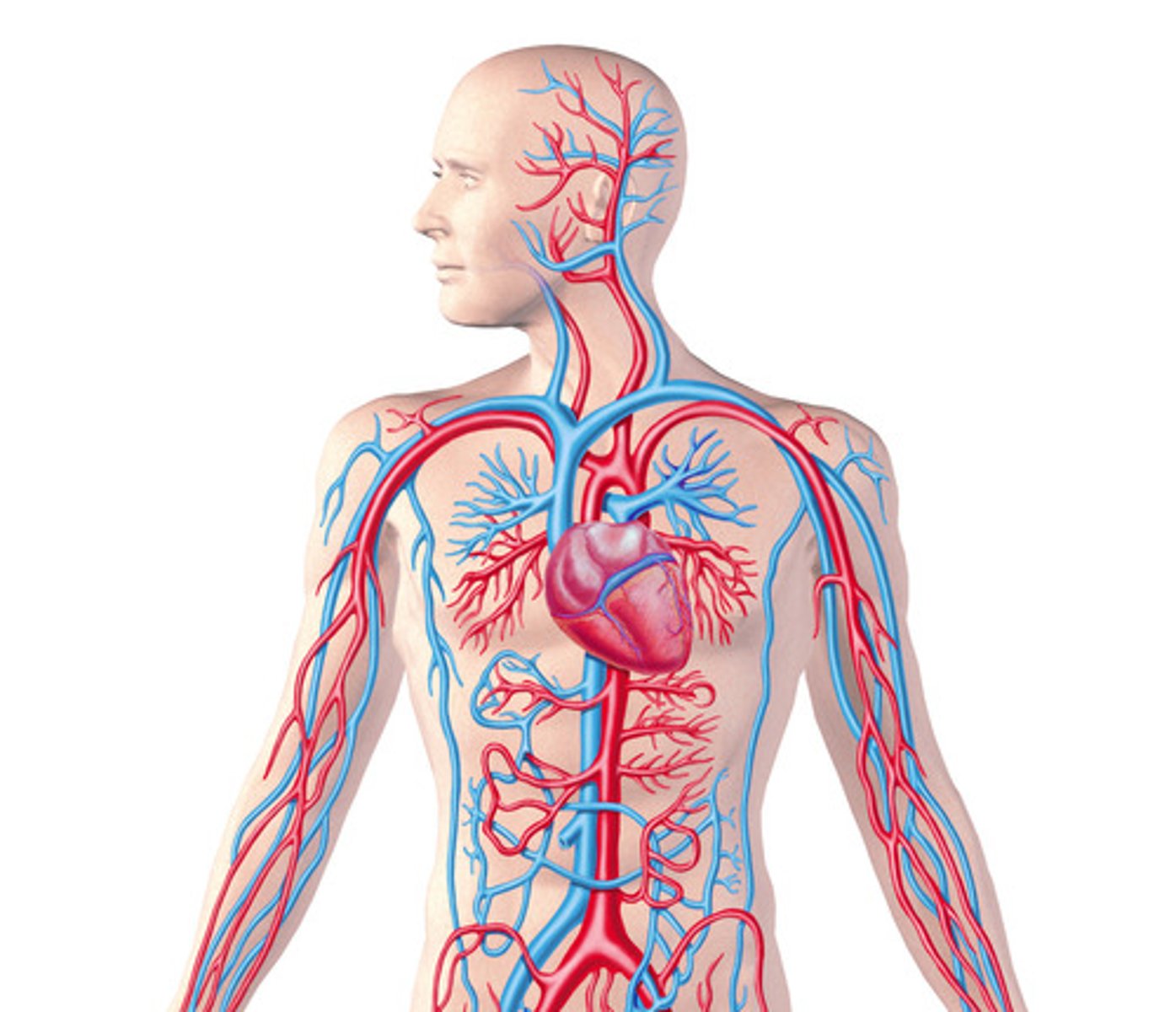
cardiovascular system
transports blood, nutrients, gases, and wastes, defends against disease, helps control temperature, fluid, and pH balance
lymphatic and immune systems
help control fluid balance, absorb fats, defend against infectious disease
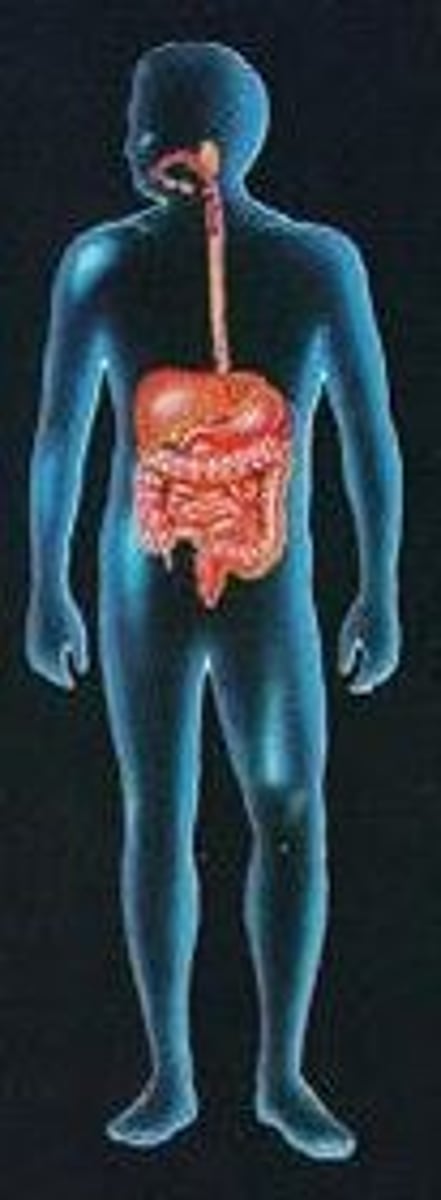
digestive system
ingests food, digests food, absorbs nutrients, eliminates waste
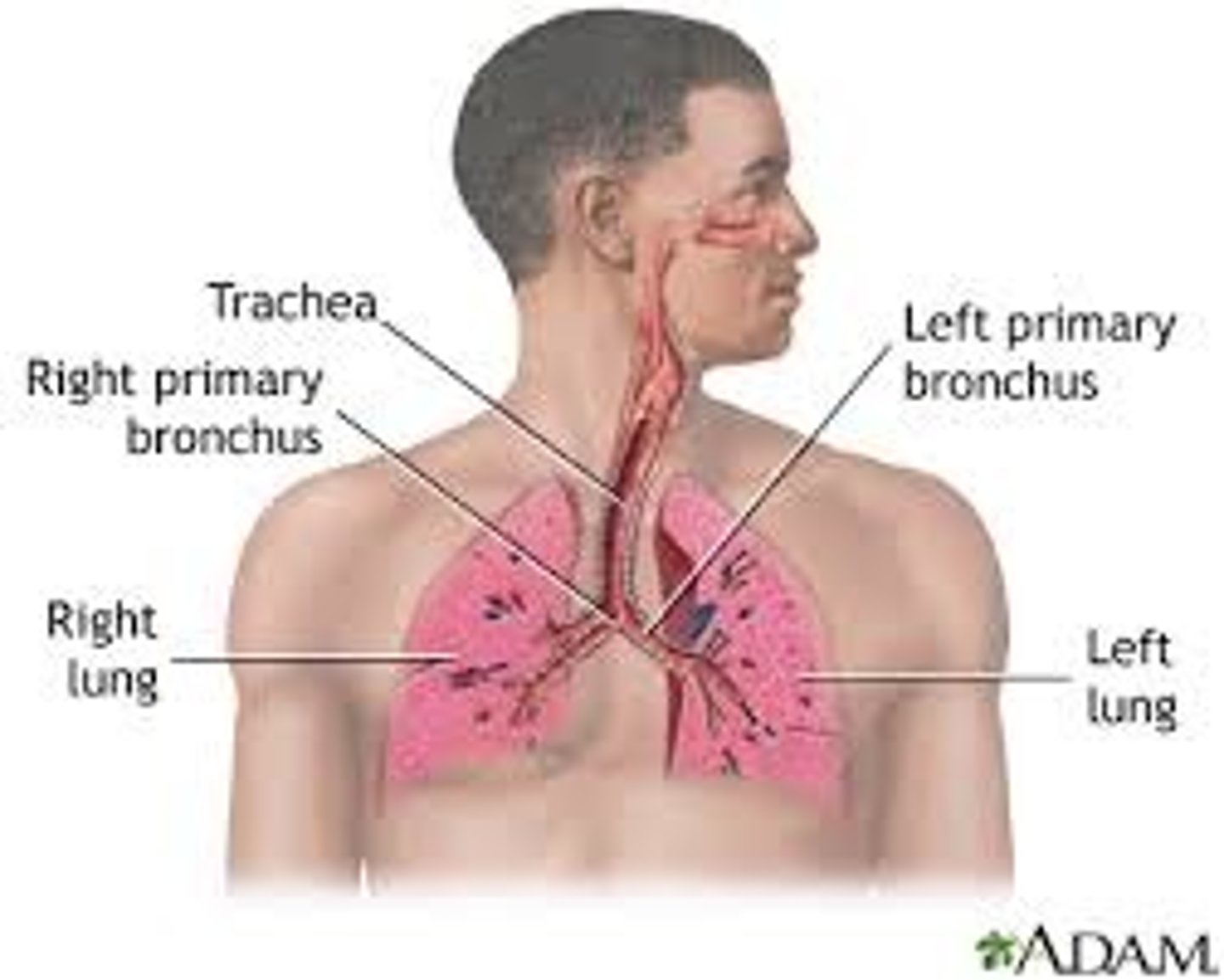
respiratory system
maintains breathing, exchanges gases at lungs and tissues, helps control pH balance