6.3/ OZ 3- Radiation and Radicals (Part 1)
1/91
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
92 Terms
At atmospheric pressure how thick would a layer of all the ozone in the atmosphere be?
Would create a layer only 3 mm thick around the Earth's surface
Why are there only small amounts of ozone in the atmosphere?
Because it quickly reacts with other substances and gets destroyed
If ozone is quickly removed by reacting why has all of the ozone in the atmosphere not been used up yet?
As some reactions produce it too
Where is the ozone layer found?
In the stratosphere which is a layer in the atmosphere
What is used to measure stratospheric ozone from the ground, how?
The Dobson Spectrophotometer- used to measure stratospheric ozone from the ground by measuring the relative intensities of UVB ration that reaches the Earth and comparing it to that of UVA radiation at ground level
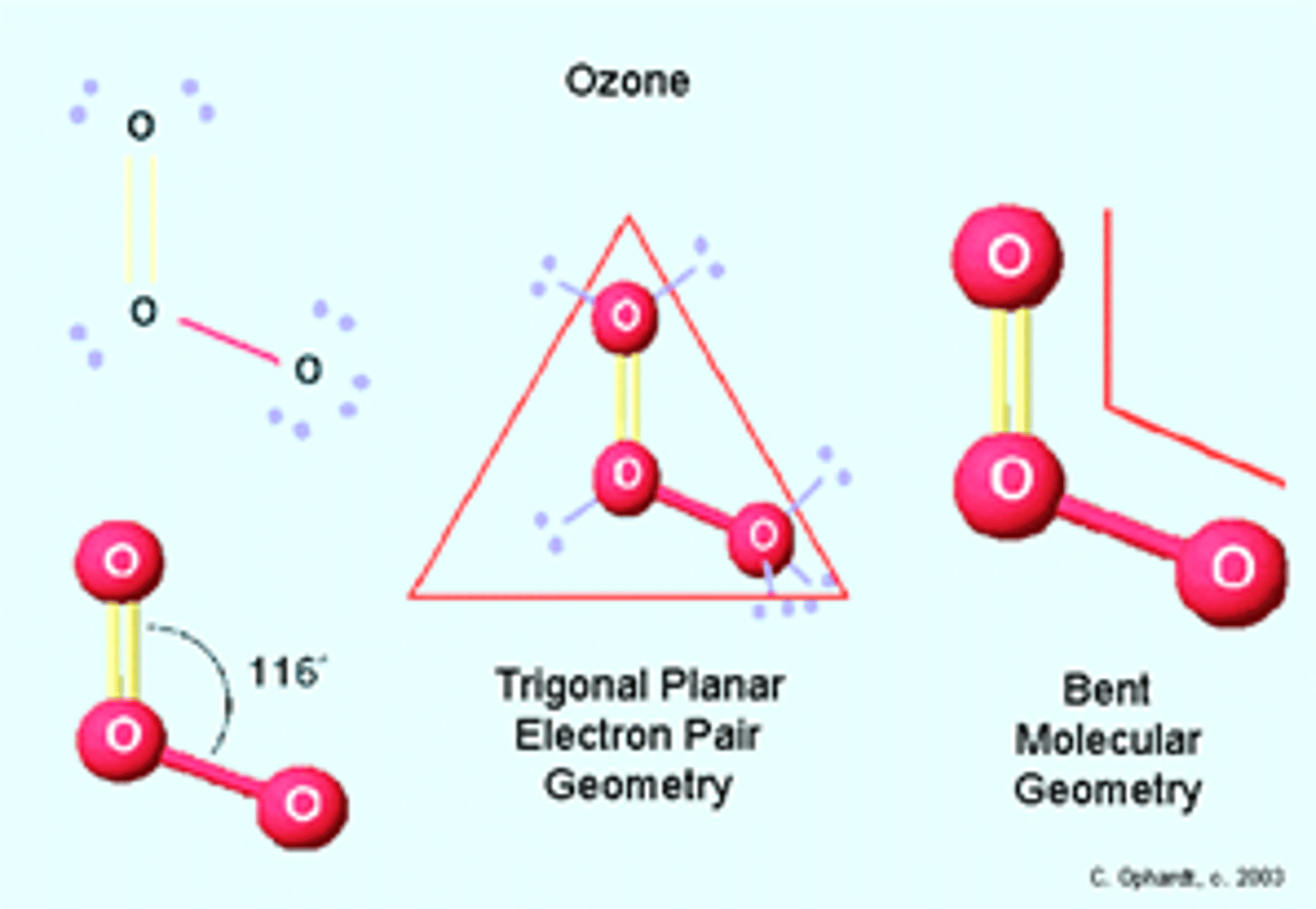
Describe the structure of the ozone molecule?
Ozone is a molecule consisting of three oxygen atoms
What shape is an ozone molecule?
Ozone molecules have a trigonal planar/ planar triangular shape
1. Central atom- the oxygen atom
2. Number of regions of charge around it- 3 (one O-O, one O=O and one lone pair)
3. Bonding angle- 120o
4. Number of lone pairs- 1
5. Corrected bonding angle- 120-2.5= 117.5o
Define bond fission
Breaking covalent bonds
What is a covalent bond?
Pairs of electrons is shared between two atoms
What is a single covalent bond?
One shared pair of electrons between two atoms
What happens to the electrons during bond fission?
Bond breaking involves the redistribution of the electrons in the covalent bond
In what two ways can a covalent bond be broken?
Heterolytic fission or homolytic fission
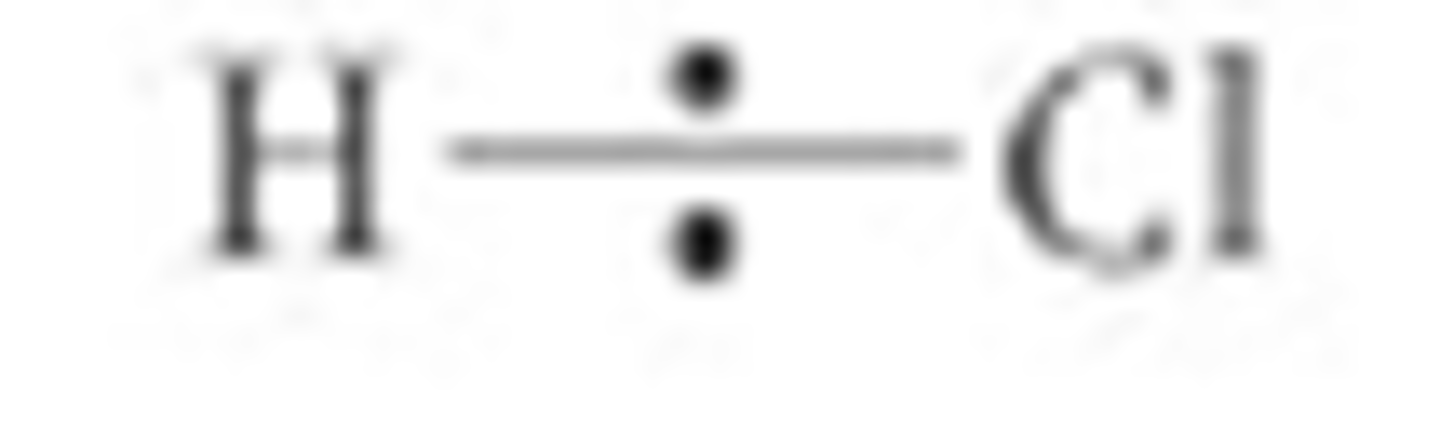
How do you show the shared pair of bonding electrons in a covalent bond?
By putting two dots over the bond
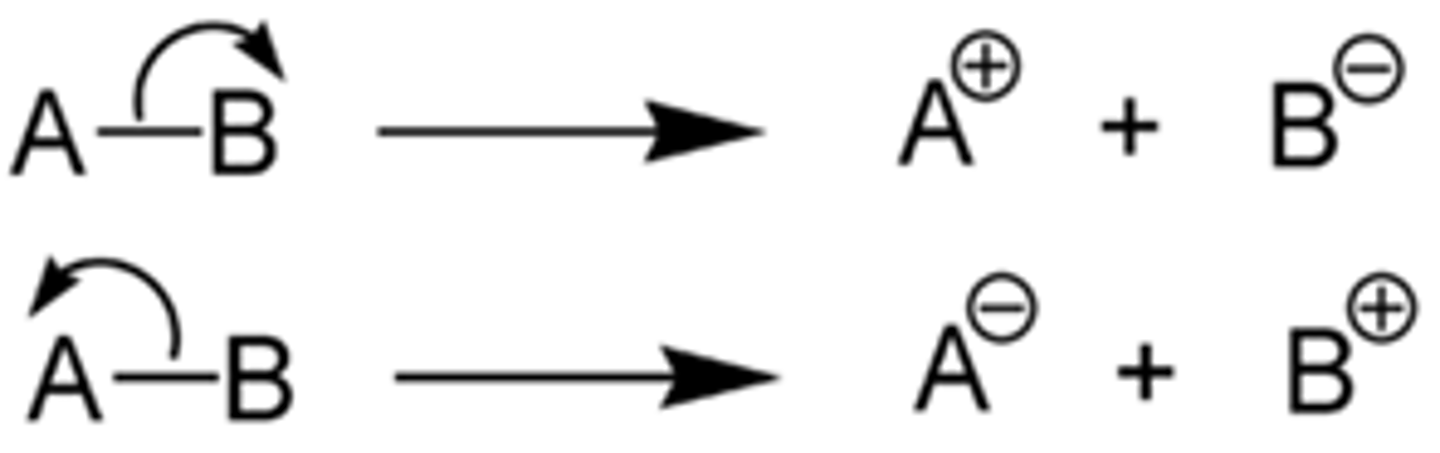
What is heterolytic fission?
One bonded atom takes both of the shared pair of electrons
What is produced in heterolytic fission, explain how?
Two ions
1. The atom that takes both shared electrons becomes a negatively charged ion (anion) because it has one more electrons than it has protons
2. The atom that does not take the shared electrons becomes a positively charged ion (cation)
Diagram- Put the two dots on the covalent bond
When does heterolytic fission usually happen?
Heterolytic fission often happens when a bond is already polar (the electrons in the bonding pair are already pulled closer to one of the atoms in the covalent bond) so the atom which is the most electronegative becomes the anion
Give an example of a bond which undergoes heterolytic fission and draw a diagram?
This happens with hydrogen-halogen covalent bonds as they are already polar
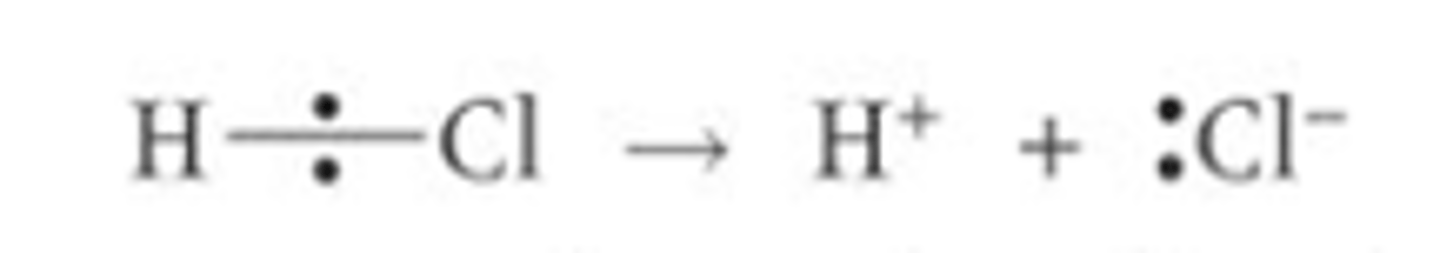
Define homolytic fission?
Each bonded atom takes one of the shared pair of electrons, so each atom has an unpaired electron and becomes a radical
What is produced in homolytic fission?
Two species of the same type are produced (two radicals)
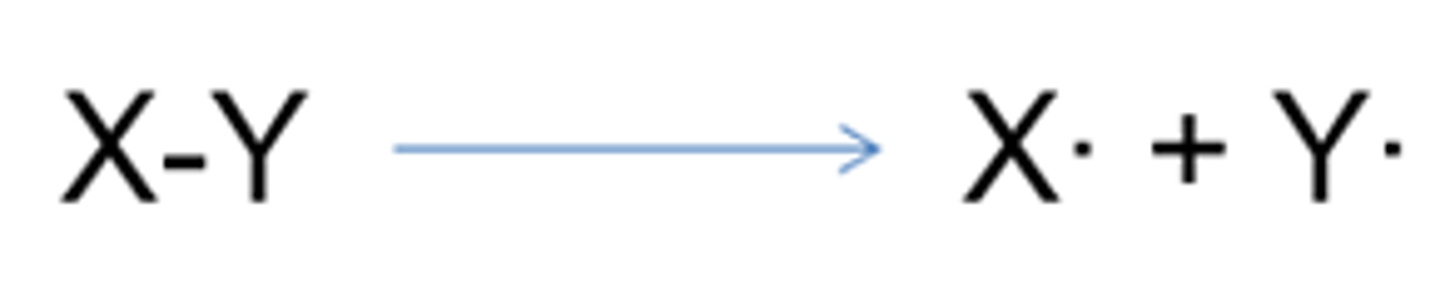
What does a dot next to a chemical symbol mean?
It shows a radical- The dot shows an unpaired electron (sometimes the dot is left out)
Do radical atoms have an overall charge?
The atoms have no overall charge because they have the electronic structure they has before they shared their unpaired electron to form the covalent bond
Why are radicals very reactive?
The unpaired electron has a strong tendency to pair up again with another electron from another substance to give the radical a full outer shell making the radical very reactive
When does homolytic fission occur?
Radicals are most commonly formed/ homolytic fission occurs when the bond been broken is non-polar so both the atoms have similar electronegativities (such as with diatomic molecules)
Give a diagram for the homolytic fission of a Br-Br bond

Explain when a polar bond may break by homolytic fission, what is this called?
Many polar bonds can break this way as well especially if the reaction is occurring in the gas phase and in the presence of light (photodissociation)
What facto decides the amount of energy it takes to break a bond by homolytic fission?
The bond enthalpy of the bond being broken
How does the amount of energy needed to break a carbon-halogen bond change depending on which halogen is present?
Carbon-halogen bonds C-F is the strongest, C-I is the weakest
Photodissociation of a C-F bond requires higher energy (shorter wavelength/ higher frequency) electromagnetic radiation or light than is required for the photodissociation of a C-I bond
Define a radical
A species with at least one unpaired electron (1 mark)
Are radicals electrically charged or uncharged?
Uncharged
Exam Question: Define the term homolytic bond fission. (1 mark)
Breaking a covalent bond evenly so that one electron goes to each atom involved in the original bond.
Exam Question: Show using a diagram the homolytic bond fission of the Cl-Cl bond. (1 mark)
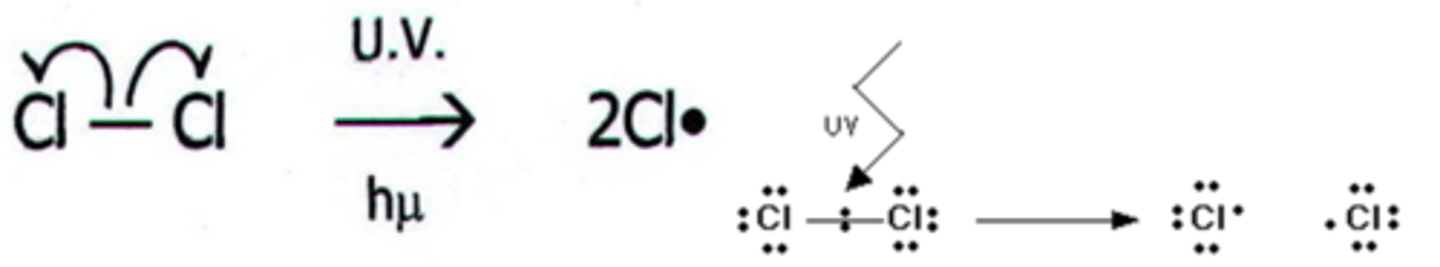
Exam Question: Define the term radical (1 mark)
A species with at least one unpaired electron
EXAM TIP- How would you determine whether or not a species is a radical?
1. Look at the dot and cross diagram to see if there is an unpaired electron
2. Count the number of outer shell electrons and if it is odd then there must be at least one unpaired electron and the species is there for a radical. E.g. CH3- C has 4 outer shell electrons, 3H is 3 outer shell electrons- total of 7 which is odd so it must be a radical
When are outer electron shells the most stable?
Filled outer electron shells are more stable than unfilled ones
Why are radicals reactive?
Because they have an unpaired electron and so they need to get another electron to become more stable and fill their outer shell. They have an unfilled outer shell and they tend to try to fill their outer shells by grabbing an electron from another atom or molecule.
Define a radical chain reaction
A reaction involving three stages: initiation, propagation, termination
What do curly arrows show?
The movement of electrons
· “tail” shows where the electron starts
· “head” shows where it finishes
What are the two types of curly arrows and what do they mean?
Half- headed- single electrons
Double head/ full headed curly arrow- pair of electrons moving
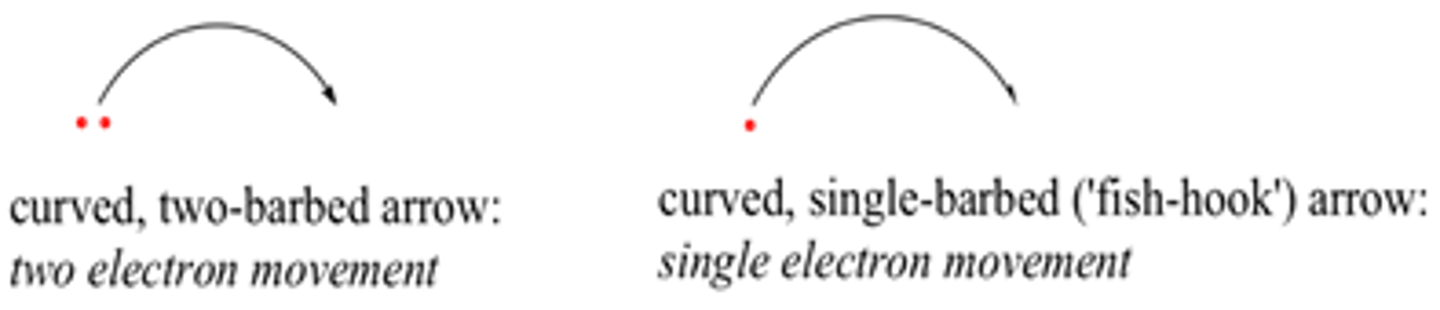
What are the three stages of a radical chain reaction?
Initiation
Propagation
Termination
Define initiation reaction
The first step in a radical substitution, initial formation of the radical by photodissociation (homolytic fission) on exposure to ultraviolet radiation/ another method, free radicals are produced
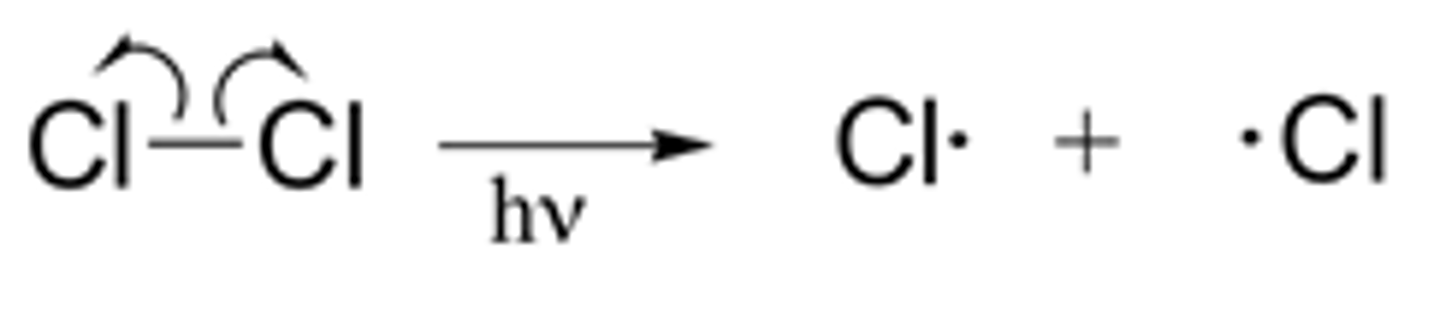
Describe the initiation reaction for the formation of the HCl
Chlorine radicals are initially formed by the photodissociation of chlorine molecules by homolytic fission. As they are radicals they are highly reactive and quickly react with another substance- initiate a reaction.
(UV over the arrow)
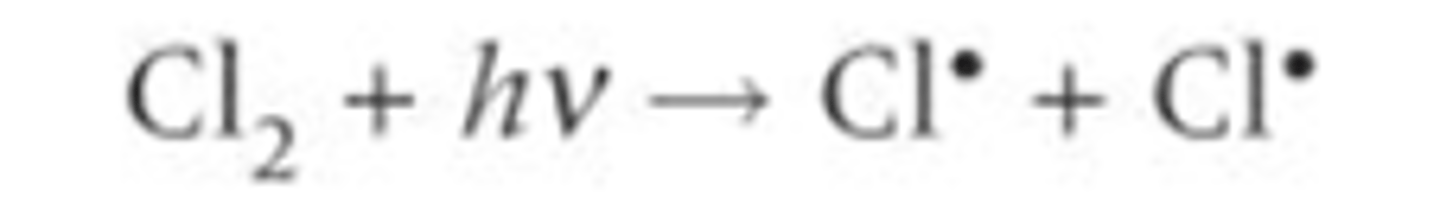
Define propagation reaction
The free radical formed in the initiation reaction then reacts with another molecule forming another new free radical which then reacts with another molecule producing even more radicals. These reactions produce new radicals which keep the reaction going- they propagate the reaction and create a chain reaction.
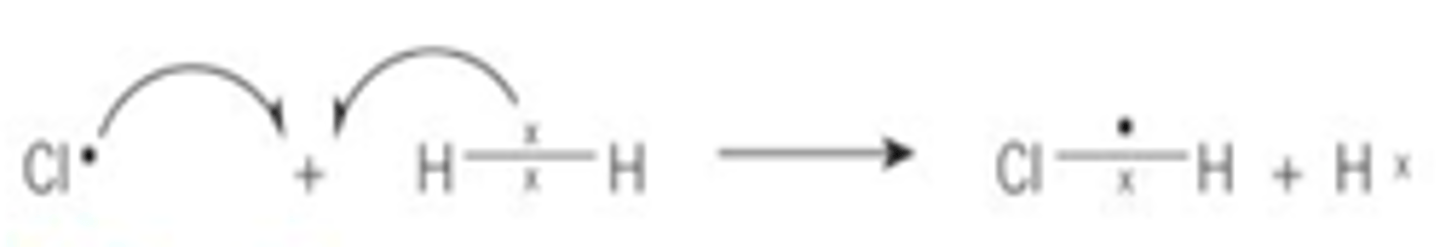
Describe the propagation reaction for the formation of HCl, give a diagram?
Chlorine radicals collide and react with a hydrogen molecule
1. The chlorine takes an electron from the pair of electrons in the bond between the hydrogen atoms this forms a new bond between the chlorine and hydrogen atoms
2. It also forms a hydrogen radical which is highly reactive and then goes on to react with chlorine molecules
3. The cycle repeats as a chlorine radical is formed
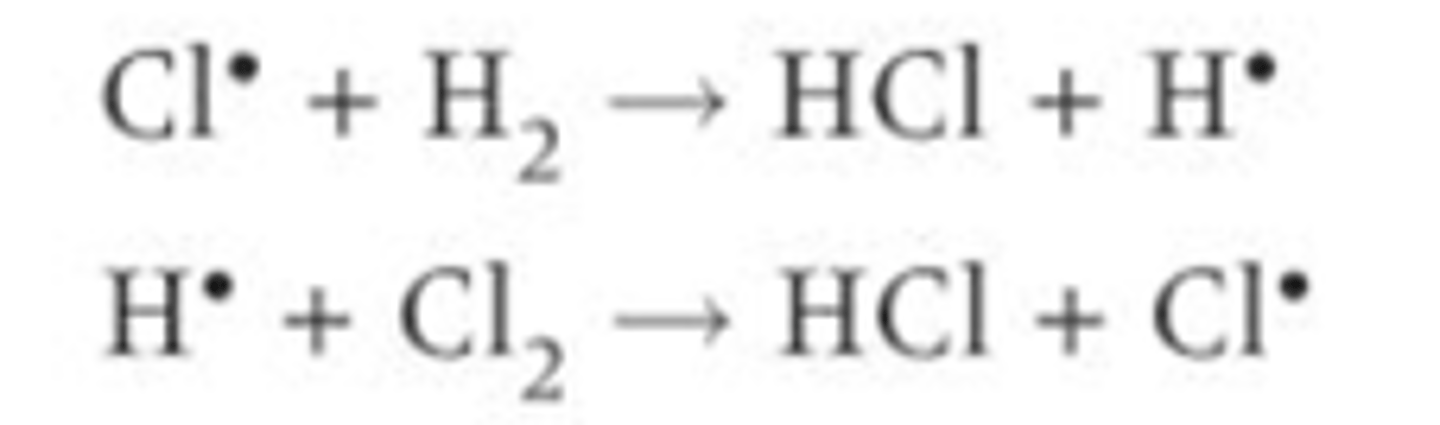
EXAM TIP- Should he number of radicals on either side of a propagation reaction be the same or different?
The number of radicals on either side of the arrow in a propagation reaction should be the same so the number of radicals in maintained
Define termination reaction?
When two radicals collide with each other and the radicals react and form a stable molecule. The radicals are removed so the chain reaction ends. The reaction is terminated because the radicals have been removed.
Describe some of the possible termination reactions for the formation of HCl
There are a number of possible combinations as there are different radical combinations
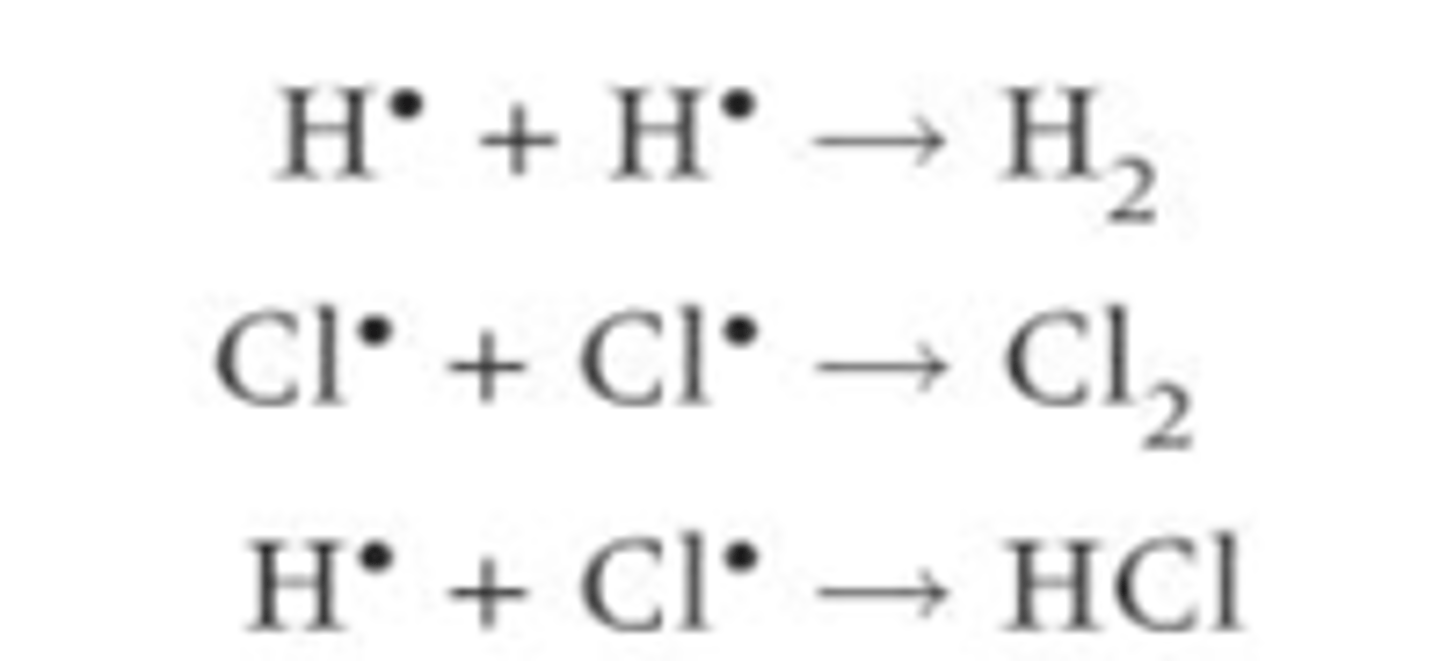
How can you produce an overall equation for the formation of HCl?
You can put all of these reaction equations together and cancel out the radicals/molecules on both sides to produce an overall equation to convert chlorine and hydrogen into hydrogen chloride
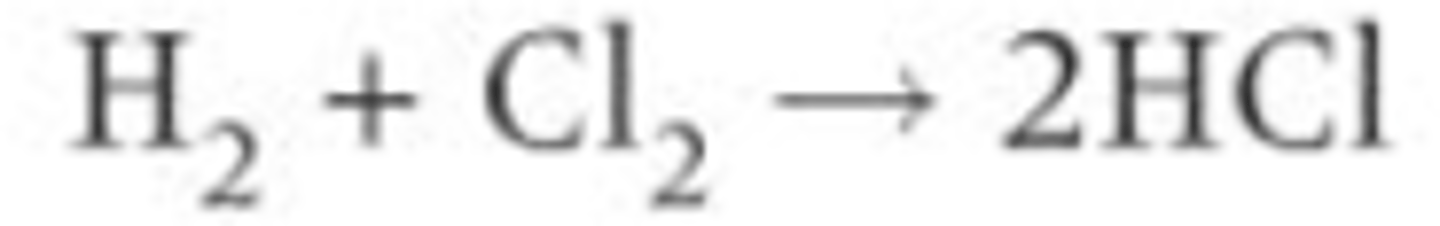
Exam Question: Why is UV light needed in the initiation step? (1 mark)
To provide energy to break the bond.
What is the energy change when a C-C bond is formed/ broken?
C-C bond broken – endothermic
C-C bond made- exothermic
What can the reaction between methane/an alkane and chlorine also be called?
A chlorination reaction but bromine and iodine will also react with alkanes in a similar way
Are alkanes usually reactive or unreactive, what will they reactive with and under what conditions?
Alkanes are generally considered to be unreactive but they will react with halogens in the presence of light/ ultaviolet radiation/ 300 degrees C- this is a radical chain reaction
Halogens and alkanes react in photochemical reactions
Define photochemical reaction
A reaction started by light
Give two reasons this is a radical substitution reaction?
1. Covalent bonds are broken by homolytic fission to form radicals with unpaired electrons
2. A hydrogen atom in the alkane is substituted by a halogen atom by a radical chain reaction.
EXAM TIP- If asked for a description of the mechanism for a reaction what should you write?
Use equations and diagrams to answer these questions. The mechanisms can be worth a lot of marks so learn them!!
Define radical substitution?
A type of substitution reaction in which a radical replaces a different atom/ group of atoms
Define mechanism
A sequence of steps showing the path taken by electrons in a reaction
What is the role of a radical in a radical substitution reaction?
They are reactive intermediates
Name the three steps in a radical substitution reaction
Initiation
Propagation
Termination
Describe the initiation step of a radical substitution reaction between chlorine and an alkane?
Two chlorine radicals are formed from a chlorine molecule
Sunlight/UV radiation? provides enough energy to break the Cl-Cl bond/ for bond fission- photodissociation
Homolytic fission- The covalent bond splits equally and each atom receives one electron from the shared pair of electrons- two free radicals are formed
After initiation the reaction can continue without the need for further energy or more radicals
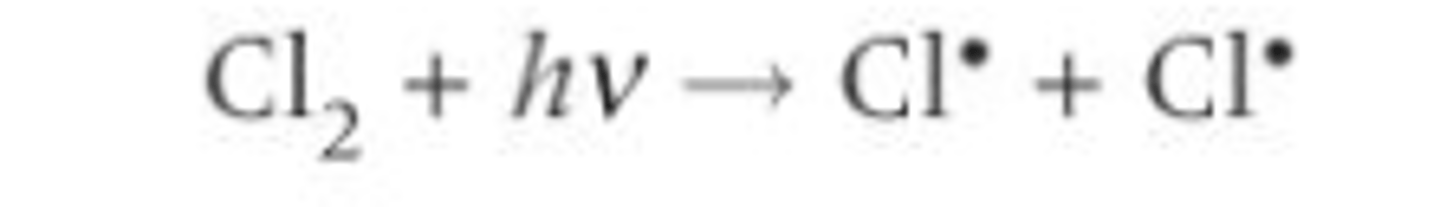
What is the first step in the propagation reaction between chlorine and methane?
Chlorine radicals react/ attack with methane molecules forming a haloalkane (HCl) and a methyl radical (CH3). A single C-H bond is broken by homolytic fission forming a methyl radical.
CH4 + Cl CH3 + HCl
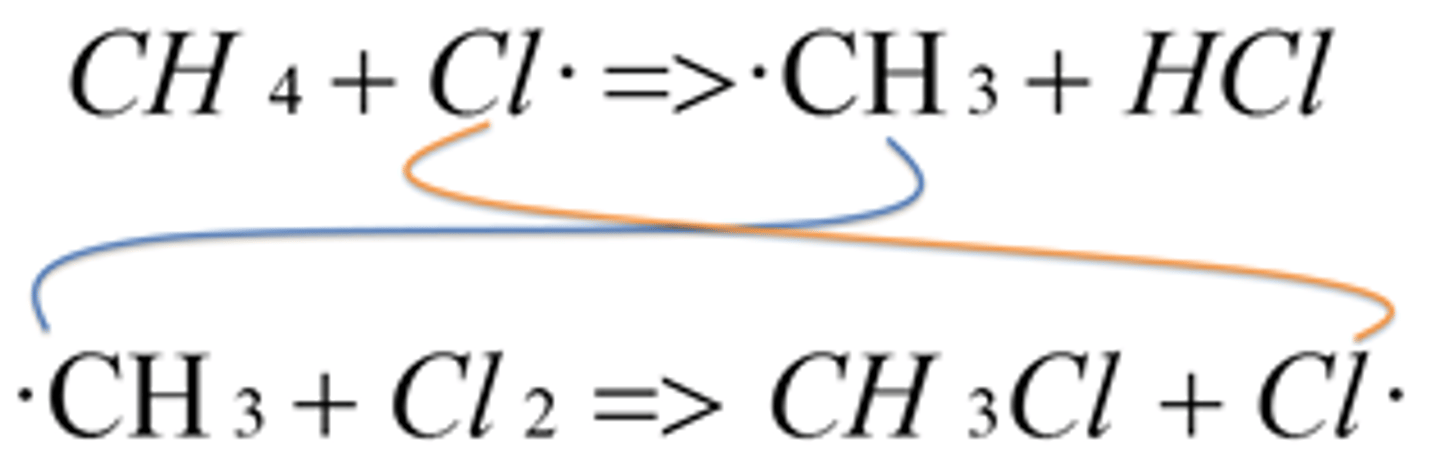
What is the second step in the propagation reaction between methane and chlorine?
SECOND STEP- The methyl radicals formed then reacts with another chlorine molecules. The organic product chloromethane CH3Cl is formed together with a further chlorine radical (regenerated) which can be used again in the first propagation step.
CH3 + Cl2 CH3Cl + Cl·
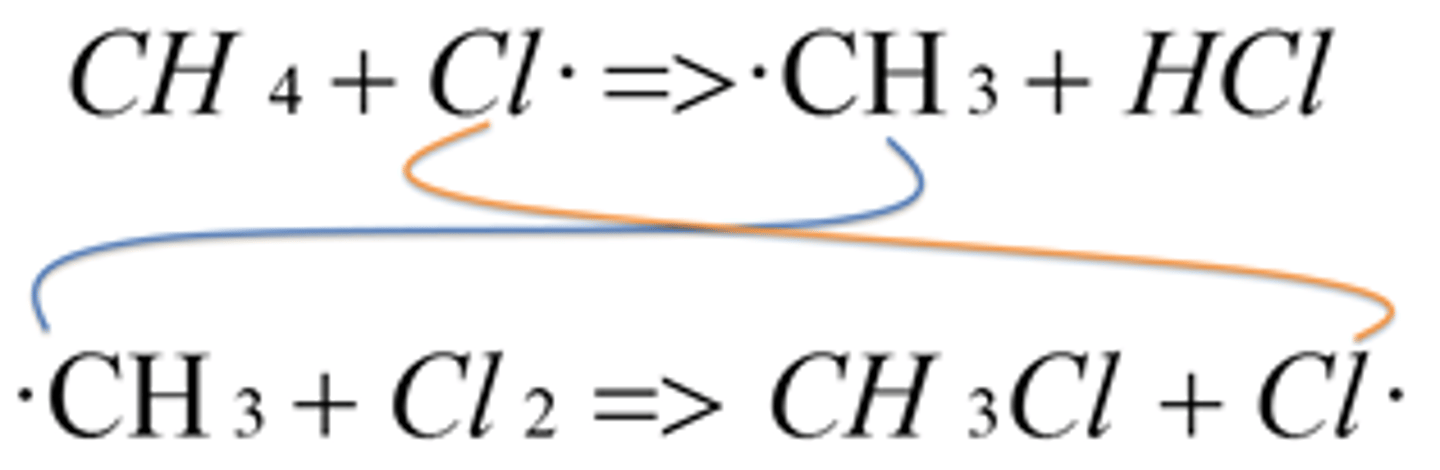
What happens after the second step in the propagation reaction between methane and chlorine
The cycle repeats- The new chlorine radical can attack another CH4 molecule. Propagation continues rapidly as a chain reaction until all the Cl2 or CH4 molecules/ reactants have reacted and none are left or the termination stage has removed all the radicals
The two repeated steps in radical substitution that build up the products in a chain reaction
What makes the propagation reaction between chlorine and methane a radical chain reaction?
A chlorine radical is used up in the first propagation step another chlorine radical is generated in the second step this can now react with another methane molecule
What is the link between the first and second steps in a radical chain reaction between chlorine and methane?
The products of the first reaction feed into the reactants of the second and vice versa this is what keeps the chain reaction going. Two steps are required to form the products. The chlorine radical is regenerated in the second step.
These two steps propagate the reaction
How do you identify the intermediates of a reaction?
"add up" the two reaction (put ALL of the reactants from both equations on one side of the equation and ALL of the products from both equations on the other side)- the intermediates appear on both sides of the reaction as they are both created and destroyed by the propagation steps
If the intermediates are cancelled out (species which occur on both sides of the reaction- you can write the overall equation for the two propagation steps)
In the reaction between chlorine and methane when will the terminations step occur?
The propagation step will have cycled through thousands of times before the termination step
What happens in the termination step between chlorine and methane, list some of the possible products?
The step at the end of radical substitution, the reaction ends when two free radicals combine to form a molecule
All of the radicals are removed stopping the reaction
There are many possible termination steps because of the large number of different combinations of two radicals
Some possible products- chlorine, ethane and chloromethane are produced If two free radicals join together they make a stable molecule
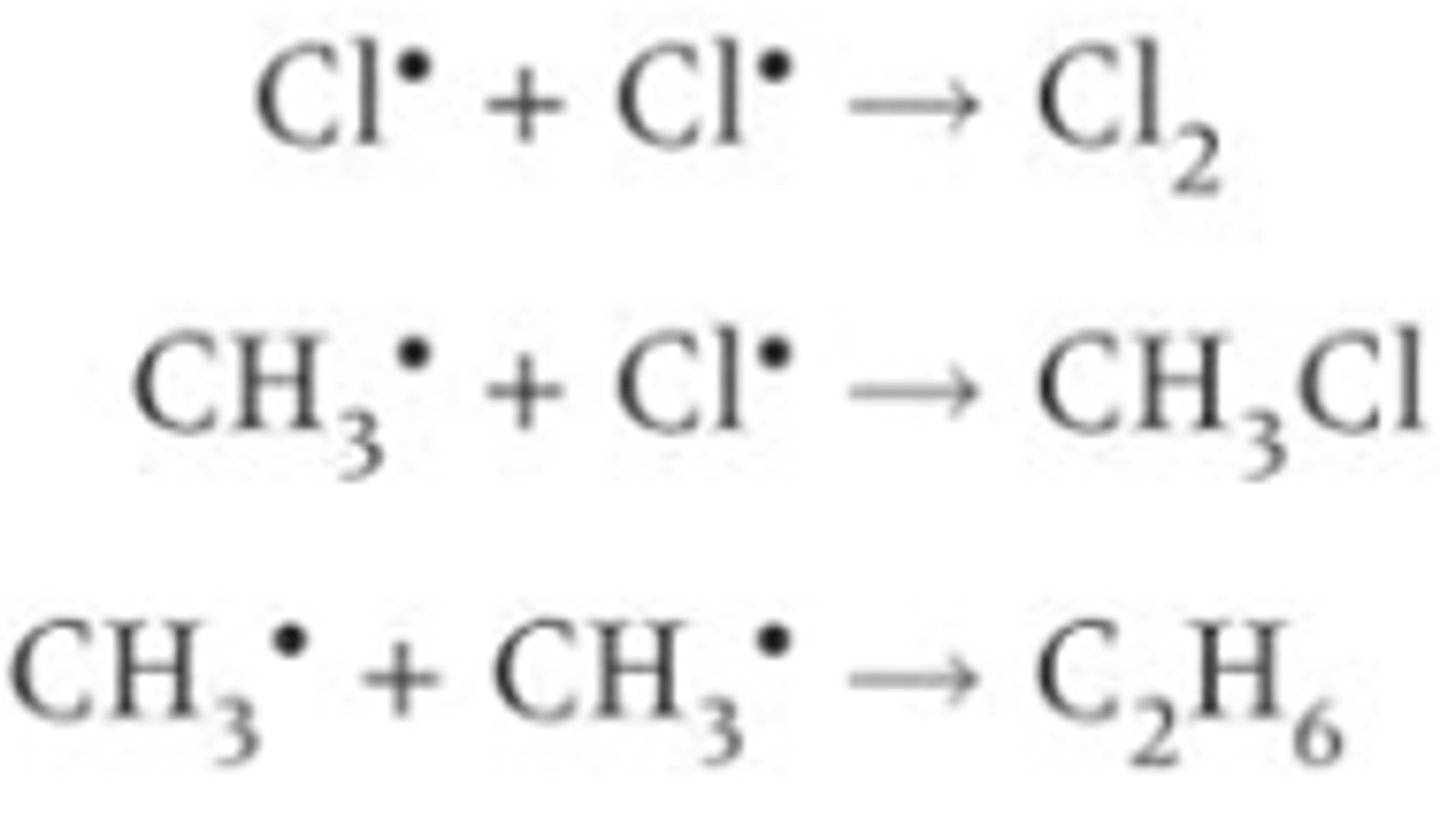
What is the consequence of a mixture of products from the termination step between chlorine and methane?
It leads to a mixture of products including other organic products being formed in radical substitution reactions. Some products formed will be trace impurities in the final sample (such as ethanol)
In a termination reaction which of the reactants/ products are radicals?
In termination reaction all of the reactants are radicals and none of the products are radicals
After the termination step between chlorine and methane what further reactions can occur?
Further substitution may occur to form dichloromethane and trichloromethane, chloromethane can further react with chlorine radicals until all hydrogen atoms are replaced.
Chloromethane from the propagation step can react with further chlorine radicals until all of the hydrogen atoms have been replaced to produce a mixture of chloromethane (CH3Cl), dichloromethane (CH2Cl2), trichloroethane (CHCl3) and tetrachloromethane (CCl4)
What are the products of a reaction between chlorine and methane?
The effect is to produce hydrogen chloride, chloromethane and small amounts of ethane
Give the overall equation for a reaction between chlorine and methane
CH4 + Cl2 ---> CH3Cl + HCl (UV over the arrow)
Exam Question: Chloropropane reacts with chlorine to make dichloropropane and hydrogen chloride
1) Give the propogation and termination reactions
2) Write out an overall equation
Propagation: Cl∙ + C3H8 --> HCl + C3H7∙
C3H7∙ + Cl2 --> C3H7Cl + Cl∙
Termination: C3H7∙ + C3H7∙ --> C6H14
Overall Equation: C3H7Cl + Cl2 --> C3H6Cl2 + HCl
EXAM TIP- when forming organic products how can different forms of the same product occur?
There may be different structural isomers of organic compounds formed (e.g. halogen atom attached to a different carbon atom)
Add reactions from the CGP book
Where are radicals produced in the body?
Cells in the human body produce tens of thousands of radicals every day
Radicals are a natural by-product of normal cell metabolism from fighting infection or burning glucose for energy
Radicals are important in hormone and enzyme development
What are the consequences of exposure to radicals?
Exposure to radicals and their reactions is linked to premature aging
Radicals can cause extensive cell damage and contribute to many diseases
Radicals damage the cell membranes of nearly all cells and they are the source of our genetic material (DNA)
What does the body produce to remove radicals?
The body produces anti-oxidants which protect the body from radicals
Name another source of antioxidants
Vitamins C (fresh fruit and veg) and E (fats, oils, nuts and green leafy veg, egg yolk and liver)
Name 4 ways we are exposed to radicals?
Cigarette smoke- smoking causes lung cancer and chronic bronchitis there are an estimated 1015 radicals in one puff of cigarette smoke including nitrogen monoxide and nitrogen dioxide radicals which interact with many biological molecules in your lungs
Sunbathing
General pollution
Food contaminated by herbicides
Give the initiation, propagation and termination reactions for a reaction between methane and bromine.
Initiation-
Br2 --> Br● + Br●
Propagation-
CH4 + Br● --> CH3● + HBr
CH3● + Br2 --> CH3Br + Br●
Termination-
Br● + Br● --> Br2
Name three key features which can be used to identify an initiation reaction
One (or more) molecules react to form radicals
A bond breaks (by homolytic fission) so the reaction is endothermic
Often happens in the presence of UV light
Name three key features which can be used to identify a propagation reaction
A molecule and a radical react to form a new molecule / radical pair
A bond breaks and a new bond forms (so you cannot easily predict whether the step is exothermic or endothermic)
Propagation reactions usually occur in pairs - one propagation step produces an intermediate that then reacts in a second step
Name two key features which can be used to identify a termination reaction
Two radicals react to form one molecule
A bond forms, so the step is exothermic- energy is released to the surroundings
What is a radical acting as if it is regenerated at the start and the end of a reaction?
A catalyst (maybe regenerated in a different propagation reaction)
Exam Question: Hydrogen and bromine gas react in a radical chain reaction. Write the equations for the initiation, propagation and termination reactions. (3 marks)
Initiation: Br2 + UV (over arrow?) ---> 2Br∙
Propagation: H2 + Br∙ ---> H∙ + HBr
H∙ + Br2 ---> HBr + Br∙ (both needed for mark)
Termination: H∙ + H∙ ---> H2
H∙ + Br∙ ---> HBr
Br∙ + Br∙ ---> Br2 (only one of these needed for mark)
Exam Question: Write out a full mechanism for the three stages of the radical substitution reaction when chloropropane reacts with chlorine to make dichloropropane and hydrogen chloride.(3 marks)
Initiation: Cl2 + UV (over arrow?) --> 2Cl∙
Propagation: C3H7Cl + Cl∙ --> C3H6Cl∙ + HCl
C3H6Cl∙ + Cl2 --> C3H6Cl2 + Cl∙ (both required)
Termination: C3H6Cl∙ + C3H6Cl∙ --> C6H12Cl2
C3H6Cl∙ + Cl∙ --> C3H6Cl2
Cl∙ + Cl∙ --> Cl2 (only one required for the mark)
Describe the method for the photodissociation of bromine experiment, draw a diagram?
Add hexane to three test tubes then add a drop of bromine to each and leave tube 1 uncovered, tube 2 completely covered and tube 3 has only the liquid covered in foil
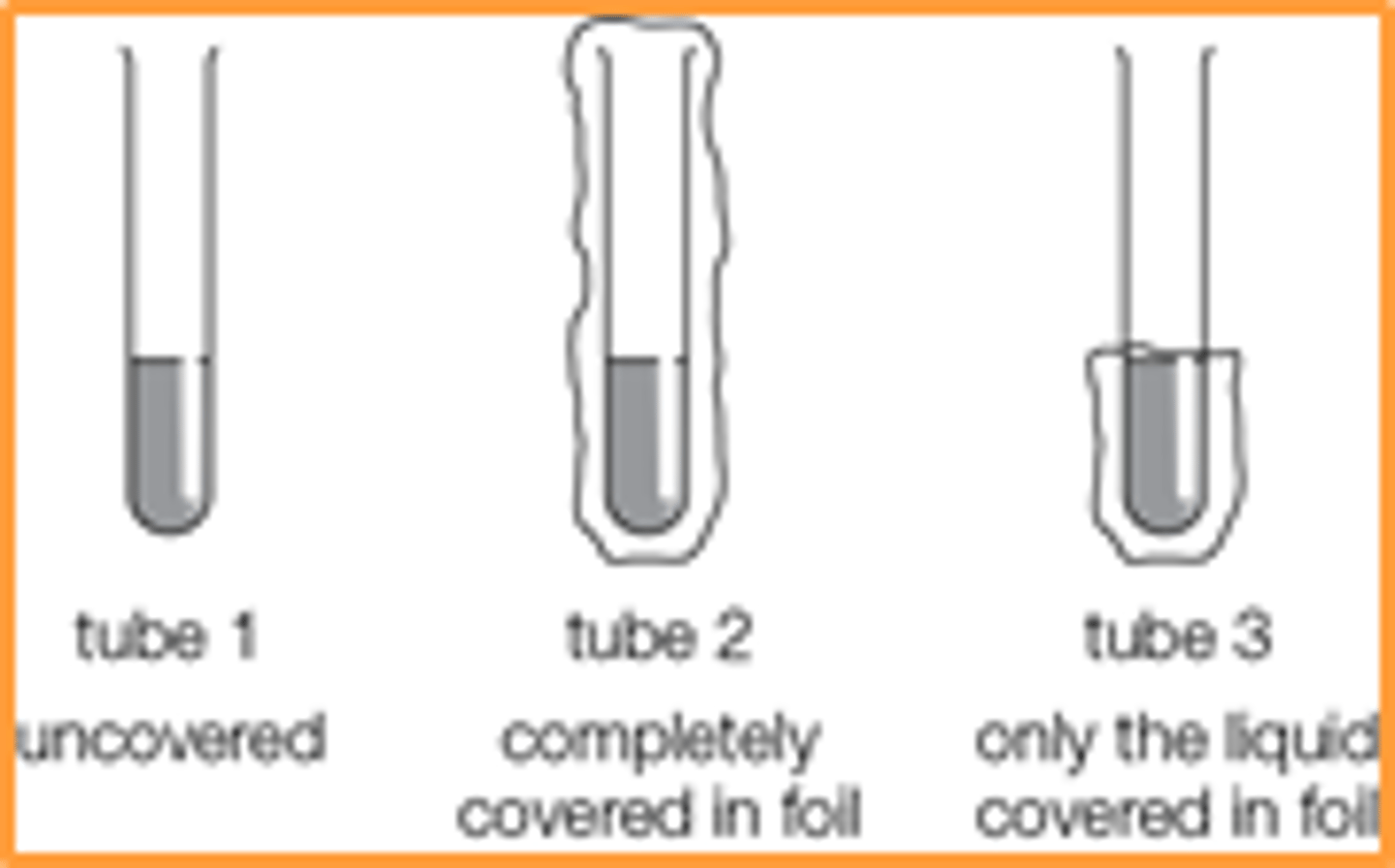
Photo dissociation of bromine experiment- what were the results for the uncovered test tube
1) Appearance of mixture after exposure to light
2) Test b blowing across mouth of tube
3) Test with universal indicator paper
4) Test with concentrated ammonia solution
1)Mixture has turned colourless
2) Steamy fumes
3) Turns red
4) White fumes
Photo dissociation of bromine experiment- what were the results for the completely covered test tube
1) Appearance of mixture after exposure to light
2) Test b blowing across mouth of tube
3) Test with universal indicator paper
4) Test with concentrated ammonia solution
1) No change. Mixture still red
2) No reaction
3) No change
4) No reaction
Photo dissociation of bromine experiment- what were the results for the partly covered test tube
1) Appearance of mixture after exposure to light
2) Test b blowing across mouth of tube
3) Test with universal indicator paper
4) Test with concentrated ammonia solution
1) Red colour has faded near the surface of the solution
2) Slight steamy fumes
3) Turns pink
4) Some white fumes
What evidence is there that bromine undergoes photodissociation
The bromine only decolourises in the presence of light (tube 1 and near the surface in tube 3). (It is reasonable to conclude that it must be Br2 which undergoes photodissociation rather than hexane, since Br2 is a coloured liquid and absorbs visible light, though this might not be obvious to students at this stage.)