Chemistry Ionic Bonding and Chemical Tests
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Atoms which lose electrons become
Positive Ions (Cations)
Atoms which gain electrons become
Negative Ions (Anions)
Pb
Cu
Fe
Ag
Zn
2+
2+
2/3+
+
2+
Ionic bonding is the
Strong electrostatic attraction between two oppositely charged ions.
Ions compounds have a
Giant Ionic Lattice Structure
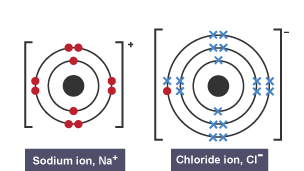
Dot and Cross Diagram
Why do ionic compounds have high melting and boiling points?
This is due to their strong electrostatic forces of attraction, which take a lot of energy to overcome.
Ionic compounds can not
Conduct electricity as solids because the ions are fixed and not free to move around.
Ionic compounds can
Conduct electricity when molten (melted) or dissolved in water because the ions are free to move around.
Test for Hydrogen
Hold a burning splint to mouth of test tube containing gas. Listen for squeaky pop.
Test for Oxygen
Relights a glowing splint
Test for Carbon Dioxide
Bubble through lime water (Calcium Hydroxide). Turns cloudy.
Test for Ammonia - ONLY ALKALI GAS
Turns Damp RED litmus BLUE.
Test for Chlorine
Bleaches damp litmus paper.
Test for Water
Boils at exactly 100 degrees.
What Flame is needed for a flame test?
A roaring blue flame as safety flame already has a colour.
Result for these Cations:
L²+
Na+
K+
Ca²+
Cu²+
Red
Yellow
Lilac
Orange/Red
Blue/Green
NH⁴+ Test
Add sodium hydroxide solution and warm. Turns damp red litmus paper blue.
Test for
Cu²+
Fe²+
Fe³+
Add sodium hydroxide.
Colour of metal hydroxide precipitate formed:
Blue
Green
Brown
Test for
Cl-
Br-
I-
Add dilute nitric acid and silver nitrate solution.
Colour of silver halide precipitate:
White
Cream
Yellow
Test for SO4²-
Add dilute hydrochloric acid and barium chloride solution:
White barium sulphate precipitate formed.
Test for CO3²-
Add dilute hydrochloric acid:
Bubble the gas given off through limewater. It should turn cloudy.
Acid is used to
destroy CO3²- ions