BIOCH 200: Slide 7 Intro Metabolism (Purpose, concepts + High E molecules)
1/77
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
78 Terms
What are the 2 Major Purposes of Metabolism?
Obtain usable chemical energy (from environment)
Make specific molecules needed to grow
What are the 2 major ways organisms obtain energy from the enviornment?
Solar E (Photosynthesis)
Consuming + breaking down nutrient molecules
Where does all E ultimately come from?
the sun
Anabolism vs Catabolism definition
Anabolism: Building larger molecules
Catabolism: Break down + Release energy
Anabolism vs. catabolism. Which is generally reductive and which is generally oxidative?
Reductive = Anabolism (electrons used to make new bonds)
Oxidative = Catabolism (electrons = removed as bonds broken)
Amphibolic pathway?
Operate in both catabolic and anabolic processes (depends on conditions)
Overall scheme/ purpose/ flow of metabolism?
Food — catabolism —> Metabolites + E —Anabolism —> Cellular constituents + E
What are the 4 dietary Macros?
Nucleic acids
Nucleotides
Proteins
A.A
Polysaccharides
Monosacch.
Triacylglycerol (fat)
F.A
Which of the 4 macros are the most significant fuel sources and which are the least?
Most = Triacylglycerol + Polysaccharides
Least = Nucleic acids
Which term best describes pathway that both consumes high energy molecules and convers monomers to polymers?
Amphibolic
Anabolic
Catabolic
Metabolic
Anabolic
What is the storage form of carbs? and what is it made of?
Glycogen: polymer of glucose
Where is glycogen stored in the body? (2 locations)
Liver (hepatocytes) = store for other tissues to use + Skeletal muscle (myocytes) = store for self to use
What are F.A. stored as? + where?
Fat (Triacylglycerol) in adipose tissue
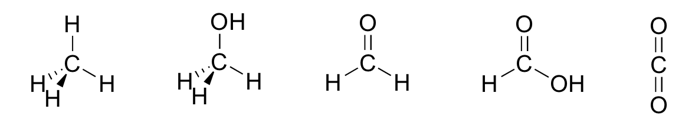
Most oxidized vs. Least oxidized
Most = CO2 (Far right)
Least = CH4 (Far left)
True or false: Fats + F.A.s are MORE REDUCED Than carbs and will generally need less oxidation steps to oxidize them?
False
Fats + FAs are more reduced
Need MORE oxidations steps to oxidize
They have a lot more electrons (and thus more E)
During catabolism what happens to the oxidation state of metabolites?
They becomes more OXIDIZED
What is typically the end product of oxidation of carbon atoms?
CO2
Anapleurotic definition
Processes that FORM/RESTORE METABOLIC INTERMEDIATES
Crucial for preserving the Equilibrium of metabolism
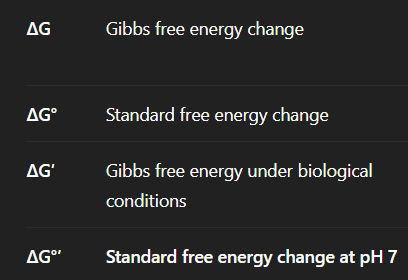
Compare + Contrast
Delta G = Actual energy change (tells if rxn will occur or not)
Delta G Naught = E change under Standard conditions (1 M (pH = 0), 1 atm + 25 degrees)
Delta G Prime = Under physiological pH = 7
Delta G Naught Prime = Under physiological + Standard conditions (1 M, 1 atm, 25 degrees + pH = 7)
True or False: A rxn will only proceed in the forward direction when the associated value of Delta G prime = negative (exergonic)
True
What does “spontaneous” means, when describing a biochemical reaction.
Can occur without energy input because Delta G prime = negative
Will the rxn proceed is Delta G prime if Greater than 0?
Rxn will not occur (in regards to the fwd rxn)
Will the rxn proceed if Delta G prime is less than 0?
Rxn will occur (spontaneous)
What direction will the rxn proceed is Delta G prime is MUCH LESS than 0? ( G «« 0)?
Fwd + IRREVERSIBLE
What direction will the rxn proceed is Delta G prime is EQUAL to 0?
BOTH WAYS (REVERSIBLE)
Why reactions with a delta G of approx. 0 are reversible?
System = close to equilibrium
any change in concentration of product or substrate may change direction of the rxn (chateliers)
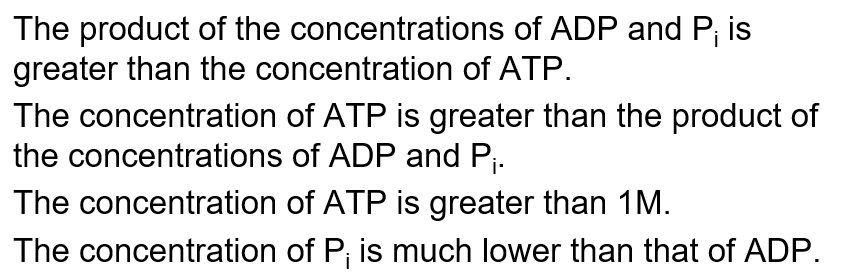
The standard free energy change (DG°´) for the hydrolysis of ATP to ADP and Pi is about -30 kJ/mol but in red blood cells the actual free energy change is about -52 kJ/mol. This means…
B
net energy is now more negative than the standard state, this means the product of the concentrations of reactants must be greater than the concentration of products (as this makes the ratio less than zero, making the second term in the equation negative
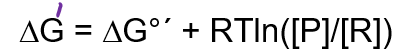
Metabolic pathway definition?
Series of ENZYME-CATALYSED chemical rxns
each individual rxn must obey thermodynamic laws
Overall rxn must also obey
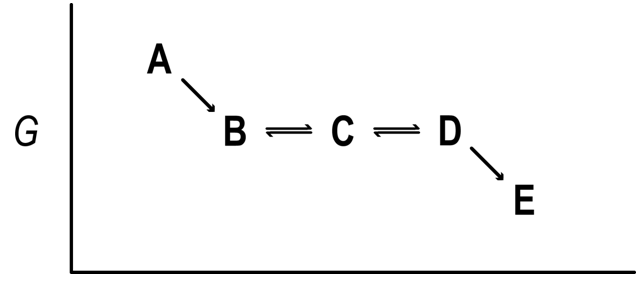
Metabolic pathways exist in a “steady state” What does this mean?
“water” flowing in = “water” flowing out + the pool stays at a CONSTANT level
The [metabolic intermediate] do not change significantly once the pathway operates
Enzyme regulation in metabolic pathways: Of Reversible vs Irreversible which rxns are typically regulated and which are not?
Irreversible = regulated
Reversible = typically not
What is the “Rate limiting step” in a metabolic pathway?
the IRREVERSIBLE regulated rxn that determines the overall rate of the pathway
3 types of regulation that can occur?
Inhibition
Activation
Reciprocal regulation
2 types of Inhibition?
Product inhibition
Feedback inhibition
What is product inhibition?
Enzyme = inhibited by the product of its reaction (immediate product)
What is feedback inhibition?
An enzyme is inhibited by a metabolite FUTHER downstream
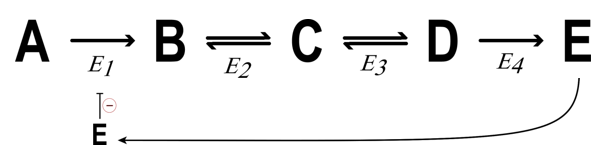
What is Feed forward activation?
Enzyme = activated by metabolite up stream
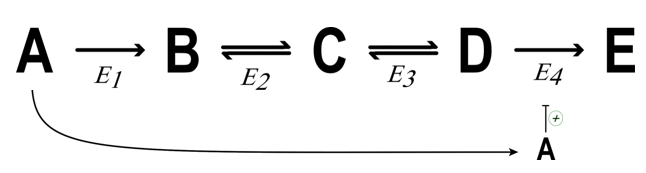
What does feed forward activation do in terms of maintaining flow/ steady state
Ensure pathway = functioning properly + in concert
prevent buildup of intermediates
What is reciprocal regulation?
OPPOSING pathways are regulated in opposite ways, so that ONLY ONE is active at a time
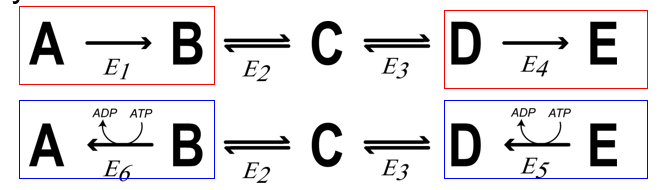
What purpose does reciprocal regulation achieve?
Ensure that both do not operate simultaneously (one = ON other must be OFF)
prevent making a molecule just to break it down again (prevent wasting E)

Which of the following is true in regards to reversible metabolic rxns?
C
What are High E Intermediates?
Compounds which contain usable chemical energy
What are the 3 major types of high E intermediates?
Electron carriers
NTP (ATP, GTP etc..)
Thioesters
Catabolism vs Anabolism: Which is oxidative which is reductive?
Catabolism = oxidative
Anabolism = Reductive
In catabolism what is oxidized and what is reduced?
Metabolites = oxidized
Cofactors (oxidizing agents) = Reduced
Cofactors = NAD+ and FAD
In Anabolism what is oxidized and what is reduced?
Metabolites = Reduced
Cofactors (reducing agents) = Oxidized
Cofactors = NADPH
What are the 4 electron carriers?
NADH, NADPH, FADH2 + FMNH2
Which of the cofactors typically act as cofactors?
NAD+ (NADH), FAD+ (FADH2) and NADPH
True or False: Nucleotides are important electron acceptors/carriers in metabolism
True
NAD (nicotinamide adenine dinucleotide)
FAD (Flavin adenine dinucleotide)
What portion of these dinucleotides (NAD, NADP + FAD) enable them to undergo reversible reduction rxns?
the NITOGEN BASE
What part of the Dinucleotides (NADH and NADPH) confers the high E? and how does it work?
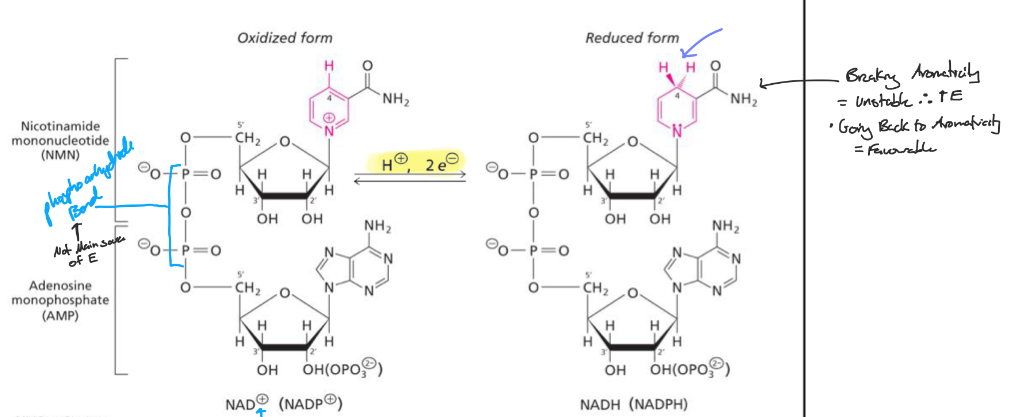
What is a cosubstrate?
Bind, converted, oxidized/reduced + released
What is a prosthetic group
Converted in place, does not disassociate. Need to reconvert in the enzyme active site where it resides
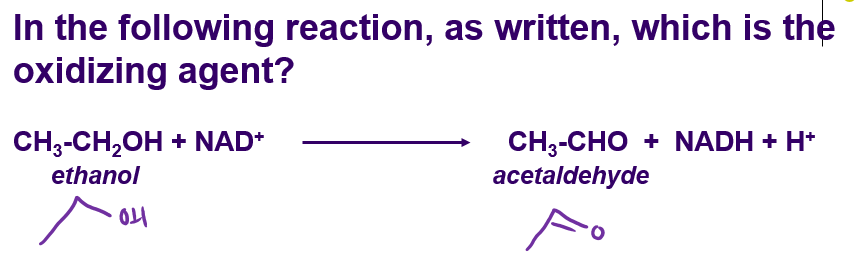
NAD+
oxidizes ethanol to aldehyde
Of the 3 high E cofactors which are cosubstrate and which are prosthetic groups
Cosubstrate = NAD+ and NADP+
Prosthetic group = FAD
How is FADH2 as a prosthetic group reoxidized back to FAD? (CAC)
Coenzyme Q
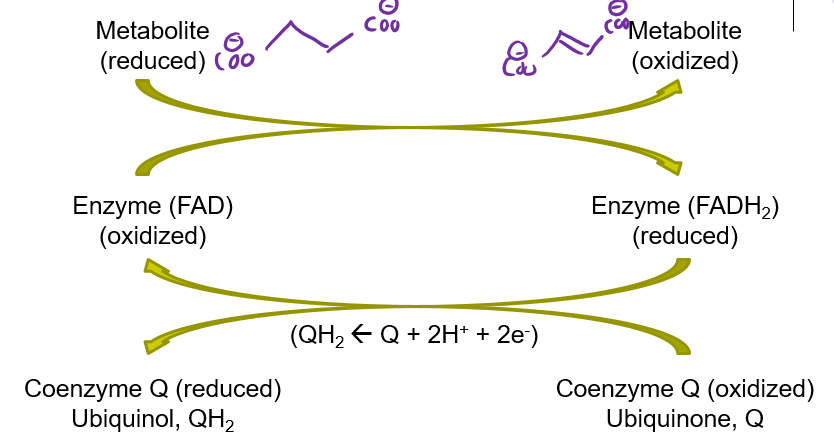
What is the function of an electron carrier?
“carry” high E electrons from one part of a rxn to another
What is the reduction of the 3 cofactors
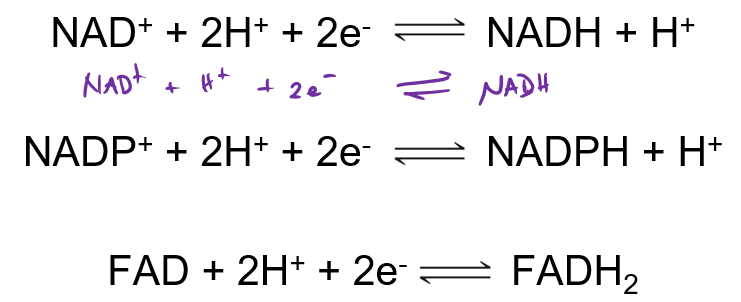
Why is ATP a high E molecule?
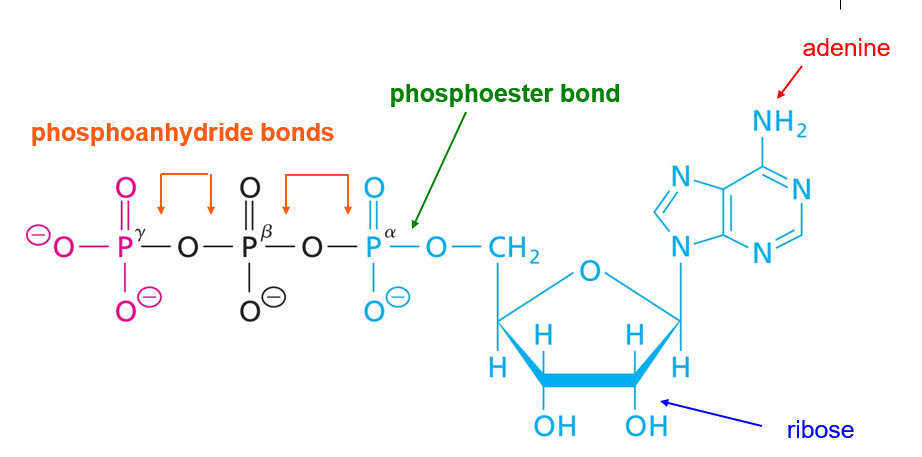
Phosphoanhydride bonds
What is a Phosphoanhydride bond?
Connection of 2 phosphates
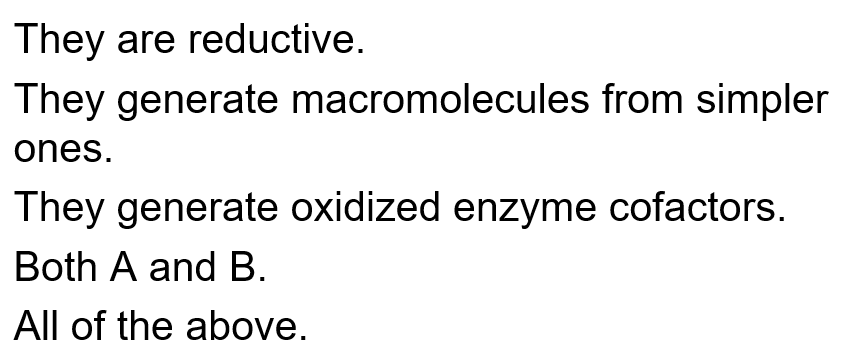
Which of the following are generally true for anabolic pathways?
E: all of the above

The reaction A + B ® C has a ∆G'° of –20 kJ/mol at 25°C. Starting under standard conditions, one can predict that:
C: As the reaction has a large negative values of deltaG, the reaction will proceed towards the formation of significant amounts of product
3 Reasons why ATP = High E molecule
3 negative charges = squished together = Electrostatic repulsion (when phosphate comes off = release tension = favorable)
Inorganic phosphate = more resonance stabilized (when ATP = broken —> stable molecule Pi = favorable)
Solvation: after ATP = broken = water can more easily surround + stabilize ADP + P)
What is a thioester?
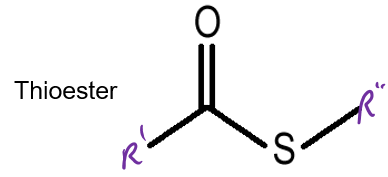

Which of the following = High E molecule
D
Both GTP and GDP have Phosphoanhydride bonds
What are 2 ways the ATP = generated by catabolism?
Substrate level phosphorylation (DIRECTLY)
Oxidative phosphorylation (Via Reoxidation of NADH + FADH2)
What are 3 uses of ATP?
Driving unfavorable rxns (coupling)
Movement (muscles flagella)
Primary Active transport (ion pumps)
Define coupling of free energies
Free energy changes = ADDITIVE
Unfavorable rxn = can occur when it occurs in concert with a favorable rxn
State the 2 conditions that must be satisfied for reactions to be coupled.
Share a common intermediate
product of one reaction is also a reactant in the next.
Overall Delta G must be negative
Define phosphate transfer potential
Free E of hydrolysis for phosphate containing compounds
some examples of phosphate transfer potential and their ranking
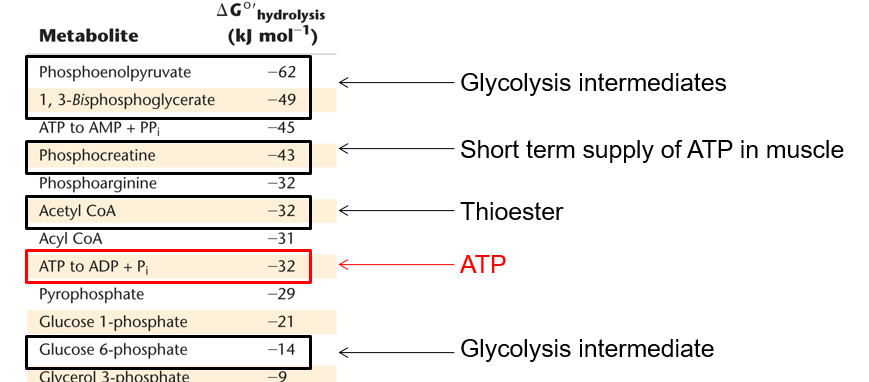
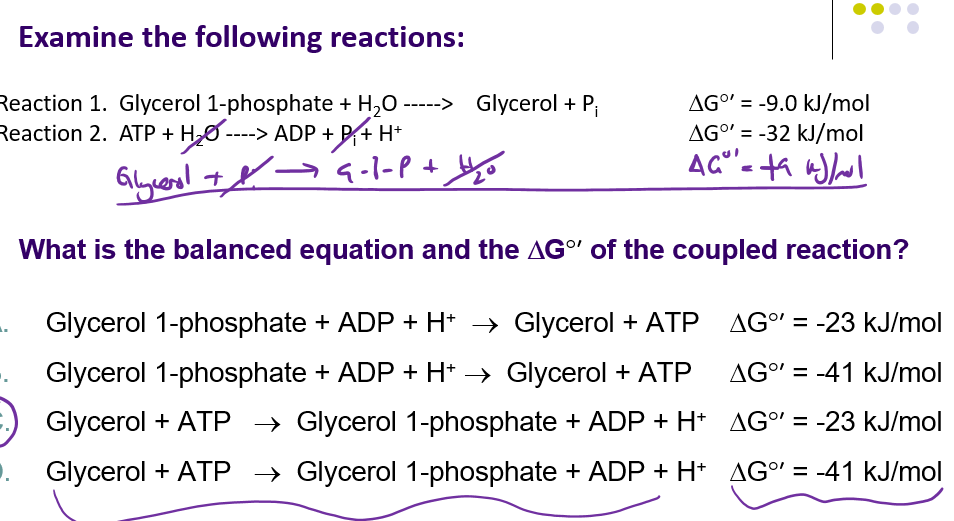
C
One reaction must be reversed, and that would be reaction 1 as it has the lower magnitude free energy change. As for rxns to be coupled they must have a negative delta g. The net free energy change is then -32 + 9 kJ/mol, or -23 kJ/mol.
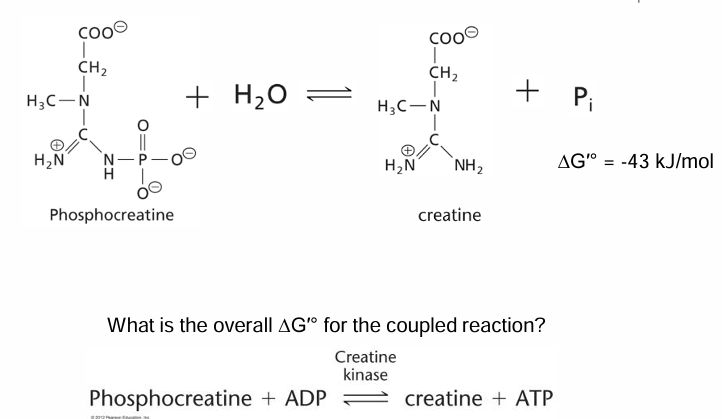
What is the Delta G naught prime?
-11 kj/mol
What is the role of phosphocreatine in muscle?
Emergency energy reserve to make ATP really fast when you suddenly need energy
short term supply

Which of the following statements concerning ATP = false?
C is false (Phosphate groups in ATP have less resonance, making them higher energy)

Metabolic pathways operate in steady states. Which of the following does not contribute to this?
C. While the statement is true, it does not relate to the concept of steady state.
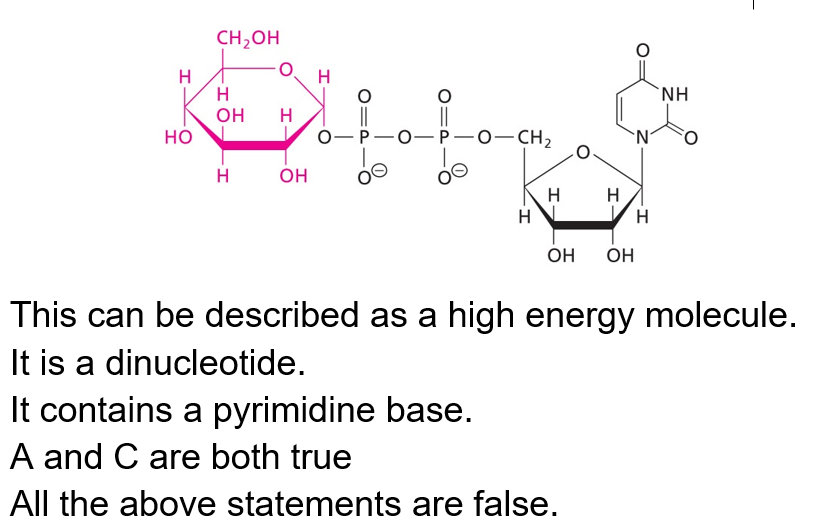
Which of the following about this structure is true?
D. This is not a dinucleotide (only one base is shown here).

D
Rxn equation of phosphocreatine use?
Phosphocreatine + ADP —> Creatine + ATP