mono, di and polysaccharides
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
what is the general formula of a carbohydrate?
(CH2O)n
what is an isomer? what are the two isomers of glucose?
compounds that have the same chemical formula but different arrangements of the atoms within the molecules and that may have different physical/chemical properties
α-glucose and β-glucose
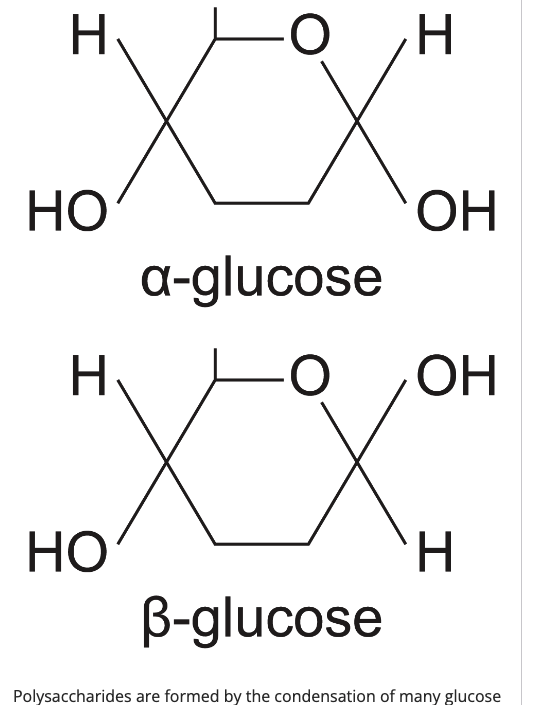
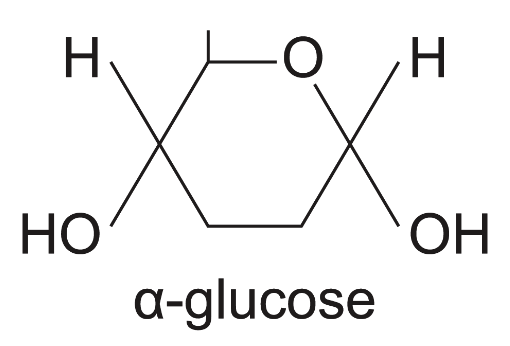
what is the structure of α-glucose?
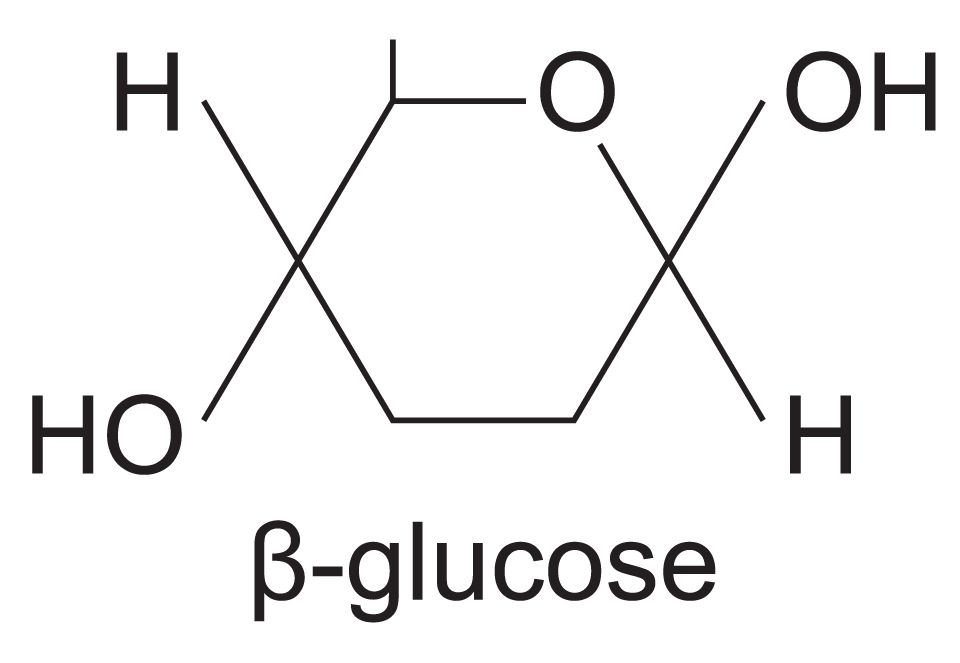
what is the structure of β-glucose?
what does a condensation reaction between glucose + glucose form?
maltose (+ water)
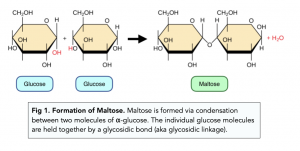
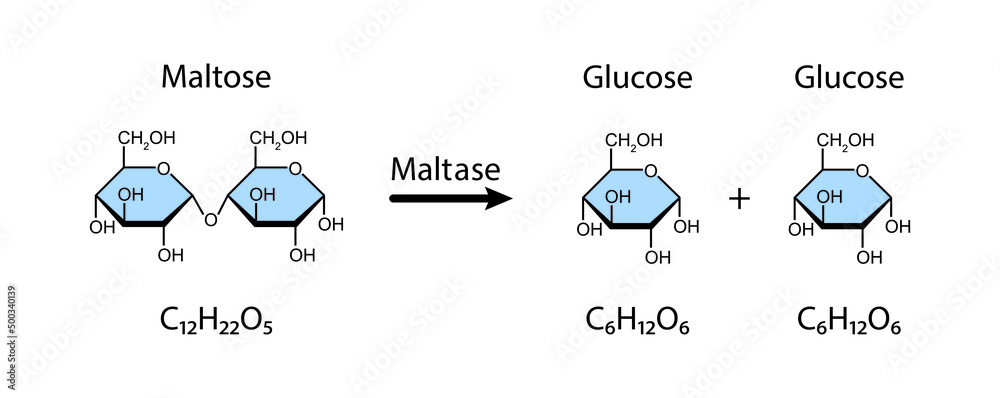
what does the hydrolysis of maltose (+ water) form?
glucose + glucose
what does a condensation reaction between glucose + fructose form?
sucrose (+ water)
what does the hydrolysis of sucrose (+ water) form?
glucose + fructose
what does a condensation reaction between glucose + galactose form?
lactose (+ water)
what does the hydrolysis of lactose (+ water) form?
glucose + galactose
what is a polysaccharide?
polymers, made up of repeating units of monosaccharides
joined by glycosidic bonds
formed by condensation reactions
what are some properties of polysaccharides?
very large
insoluble - suitable for storage
some required for structural support (e.g. cellulose)
can be hydrolysed to release di/monosaccharides
what is starch?
polysaccharide formed by the joining of ~200-1000 α-glucose molecules
starch is found in plants - in the form of starch grains in chloroplasts
its role is to act as an energy store in plants
what are thje two different forms of starch?
amylose
amylopectin
what is amylose?
contains α-1,4 glycosidic bonds
helix structure, making it the most compact form of starch (and therefore not very easily accessible for glucose release)

what is amylopectin?
contains α-1,6 glycosidic bonds (as the branches) and α-1,4 glycosidic bonds in the chain (like in amylose)
it is more branched than amylose, giving it a larger SA for faster enzyme action and ∴ faster hydrolysis
less compact than amylose, making it more easily accessible for glucose release
why is it useful that starch molecules are large?
so that they do not diffuse out of cells
why is it useful that starch molecules are compact?
they can store lots of glucose in a small space
why is it useful that starch molecules can be hydrolysed?
to form α-glucose which can be easily transported for use in respiration
why is it useful that amylopectin and glycogen have branched ends?
larger SA for faster hydrolysis due to faster enzyme action
why is it useful that starch molecules are insoluble?
no effect on water potential, i.e. no osmosis
what is glycogen? what is it made up of?
polysaccharide formed by the joining of α-glucose molecules
branched - contains α-1,6 glycosidic bonds (as the branches) and α-1,4 glycosidic bonds in the chain (like in amylose)
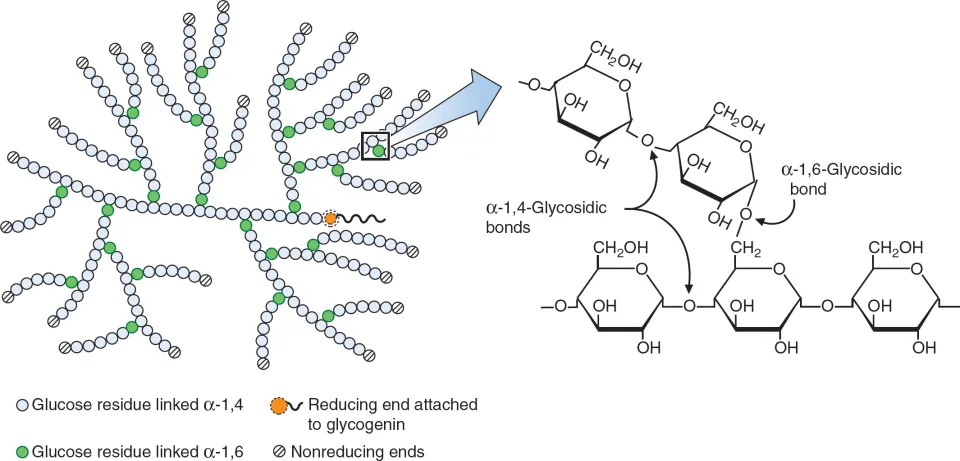
where is glycogen found?
found in animals and bacteria
in animals, it’s stored as small granules in the muscles and liver
what is the structure of glycogen?
shorter than starch, but more highly branched
as it is more highly branched, it has more 1,6 bonds, meaning it has a larger SA for faster enzyme action and so faster hydrolysis, which is more beneficial to animals as they have a more active lifestyle
what is the role of glycogen?
acts as an an energy store in animals
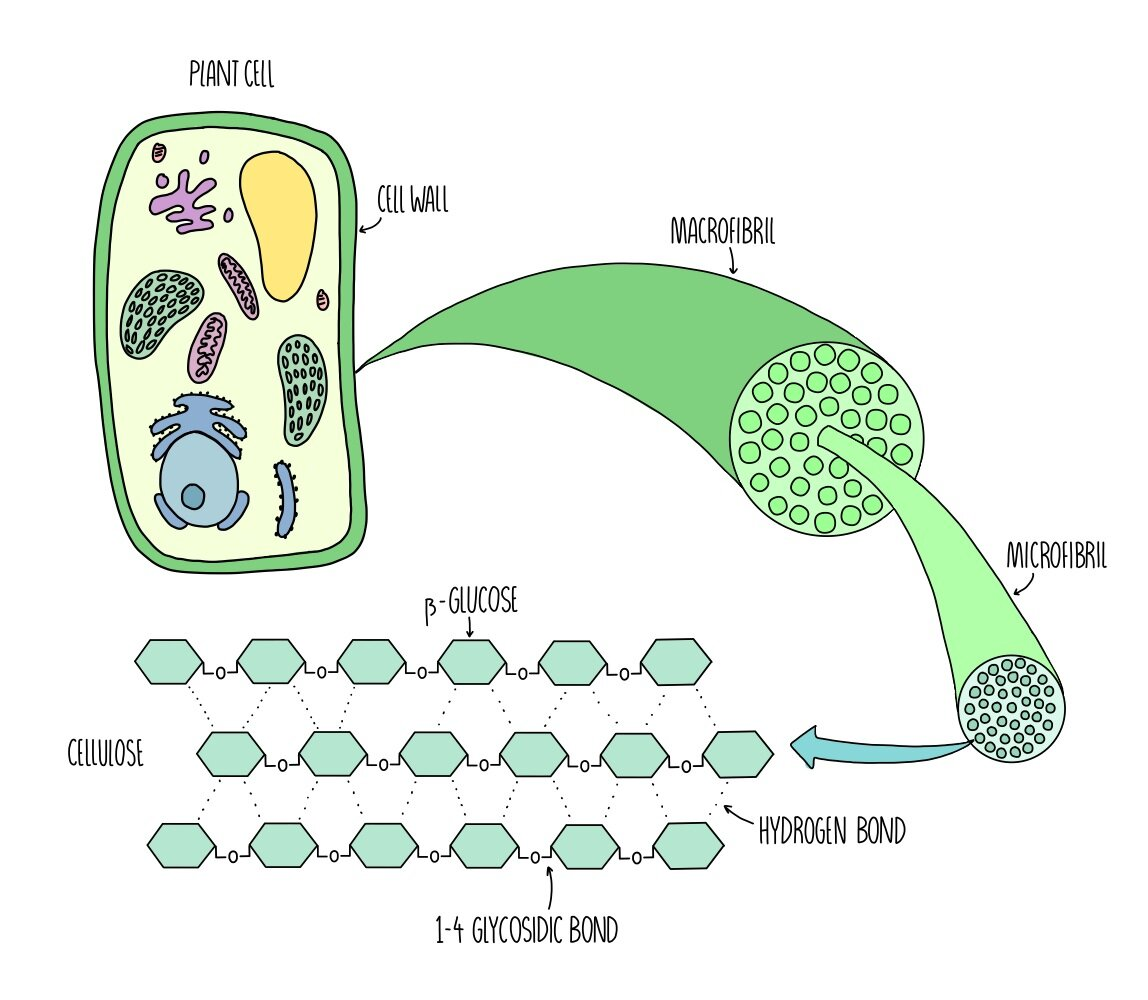
what is cellulose? what is it made up of?
polysaccharide made up of β-glucose monomers rotated 180° every two monomers
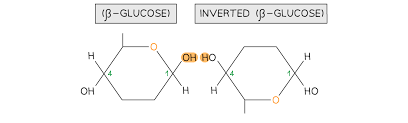
where can cellulose be found?
in plant cell walls
what role does cellulose have?
provides the structural component of cell walls
provides strength and elasticity to cells
stops cells from lysing
what is the structure of cellulose?
the straight, unbranched chains of glucose monomers interact, forming H bonds which give it strength
this is due to the chains being parallel, allowing cross linking via H bonds through adjacent chains
the interactions between the chains form a microfibril
many microfibrils form a fibre