PHYS SCI WW1 (Molecular Polarity, Intermolecular Forces, and Material Properties)
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
What are the forces that hold molecules together and influence their properties?
Molecular Polarity and Intermolecular Forces
Electronegativity Difference
Determines if electrons are shared equally or unequally between atoms.
Molecular Shape (Geometry)
Determines if the charge distribution is symmetrical or asymmetrical.
Molecular Polarity depends on…
Electronegativity Difference & Molecular Shape (Geometry)
Non-Polar Molecules
Electrons are shared equally between atoms.
Molecules are symmetrical in shape.
Examples: O₂ (oxygen), CO₂ (carbon dioxide), CH₄ (methane), fats & oils.
Polar Molecules
Electrons are shared unequally, creating partial positive and partial negative charges (dipoles).
Molecules are asymmetrical in shape.
Examples: H₂O (water), NH₃ (ammonia), sugar, alcohols.
Why do oil and water not mix?
Water is polar, while oil is non-polar. Like dissolves like, so polar and non-polar substances do not mix.
Why do perfumes evaporate quickly?
Most perfumes contain volatile polar molecules that evaporate easily due to weak intermolecular forces.
What are Intermolecular Forces (IMFs)
Determines how molecules interact with each other.
Types of Intermolecular Forces
London Dispersion Forces, Dipole-Induced Dipole Forces, Dipole-Dipole Interactions, Hydrogen Bonding, Ion-Dipole Forces
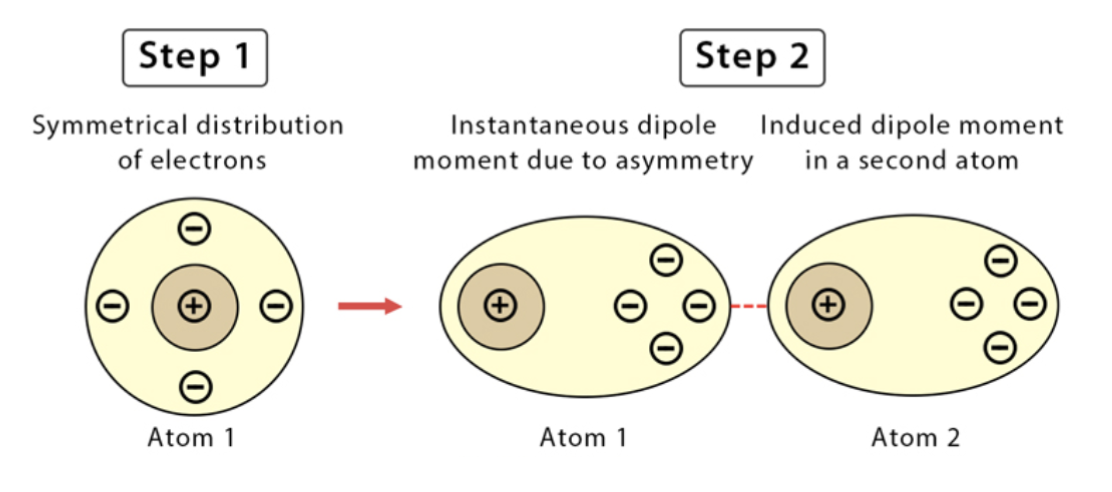
London Dispersion Forces
Present in all molecules, including non-polar ones.
Caused by temporary shifts in electron clouds, creating instantaneous dipoles.
Stronger in larger molecules due to more electrons and greater polarizability.
Weakest IMF
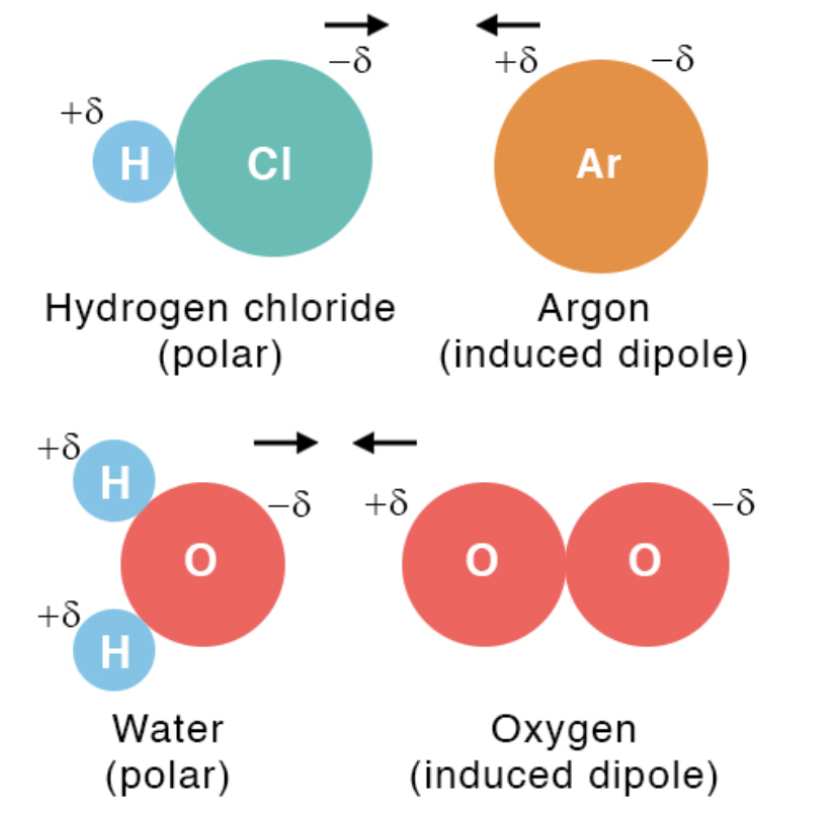
Dipole-Induced Dipole Forces
Occurs between a polar molecule and a non-polar molecule.
The polar molecule induces a dipole in the non-polar molecule by shifting its electron cloud.
Strength increases with higher polarizability of the non-polar molecule.
Dipole-Dipole Interactions
Occur in polar molecules with permanent dipoles.
The positive end of one molecule attracts the negative end of another.
Stronger than LDFs but weaker than hydrogen bonding.
Hydrogen Bonding
Special case of dipole-dipole interactions, occurring when H is bonded to N, O, or F.
Hydrogen bonding is much stronger than regular dipole-dipole interactions due to high electronegativity and small atomic size.
Strongest of the Dipole Forces
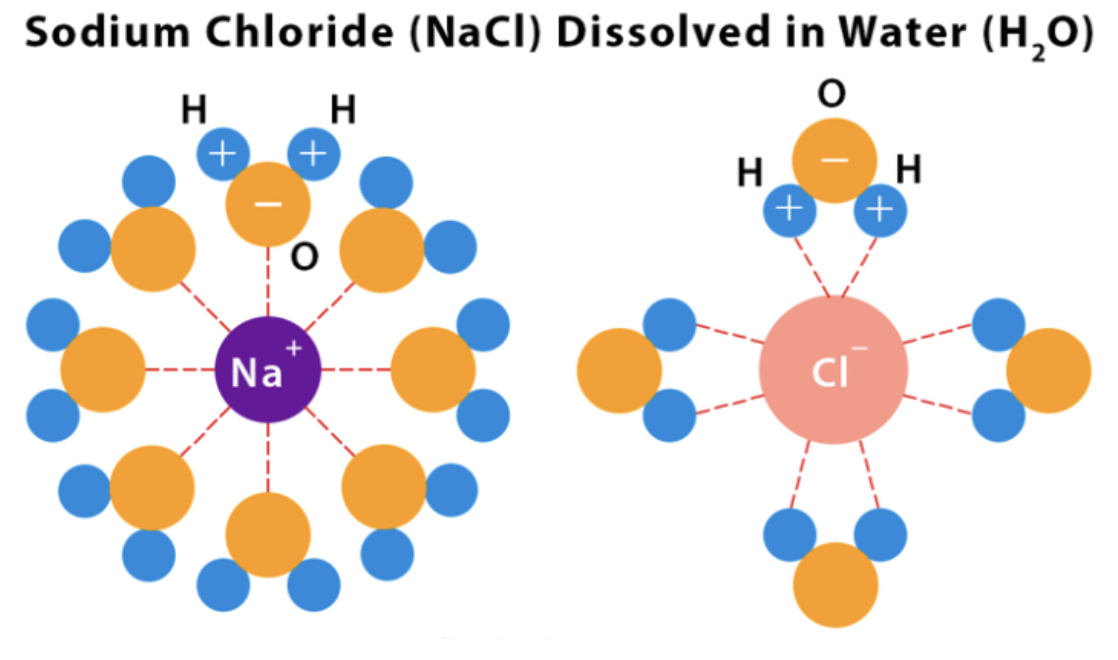
Ion-Dipole Forces
Occurs between an ion and a polar molecule (typically water).
Stronger than hydrogen bonding because ions have full charges instead of partial charges.
Plays a key role in dissolving ionic compounds in water.
Strongest IMF
Why does ice float on water?
Water molecules form hydrogen bonds that create an open lattice structure in ice, making it less dense than liquid water.
Why do oil and water not mix?
Oil = Non-polar → Only LDFs
Water = Polar → Dipole-dipole & Hydrogen bonding
Since oil lacks strong enough IMFs to interact with water, they remain separate.
Why do some perfumes evaporate faster?
Perfumes contain volatile molecules with weak IMFs (mostly LDFs & dipole-dipole interactions).
Water-based perfumes with hydrogen bonds evaporate more slowly.
How do weak IMFs affect Boiling & Melting Points of substances?
It lowers the Boiling & Melting Point of the substance (easily evaporates)
How do strong IMFs affect Boiling & Melting Points of substances?
It increases the Boiling & Melting Point of the substance (strong forces keep molecules together)
How do weak IMFs affect the Solubility of substances?
Substances dissolve in non-polar solvents
How do strong IMFs affect the Solubility of substances?
Substances dissolve in polar solvents
How do weak IMFs affect the Viscosity (thickness of liquids) of substances?
Substances flow easily
How do strong IMFs affect the Viscosity (thickness of liquids) of substances?
Substances have thicker liquid
How do weak IMFs affect the Evaporation Rate of substances?
Substances evaporate quickly due to low Boiling & Melting points
How do strong IMFs affect the Evaporation Rate of substances?
Substances evaporate slowly due to high Boiling & Melting points