Spectroscopy
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
What is a mass spectrometer?
a scientific instrument that is used to analyse the mass and structure of molecules and atoms
Summarize the process that takes place in a mass spectrometer
Vaporisation
Ionisation
Acceleration
Deflection
Detection
victory is a definitie deetection
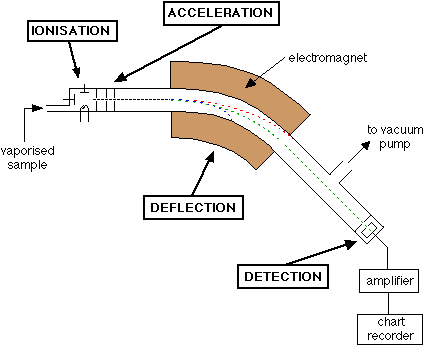
Describe the process of vaporisation that occurs in a mass spectometer
The sample (containing all the different isotopes of the element) is heated and turned into a gas before it enters the spectrometer.
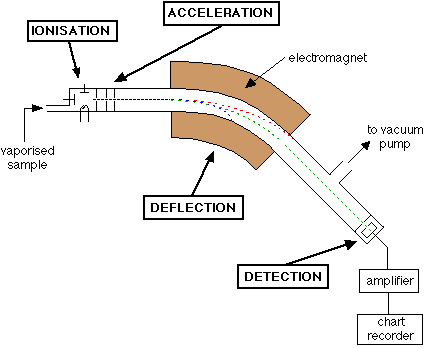
Describe the process of ionisation that occurs in a mass spectrometer
The gaseous sample is bombarded with high energy electrons.
This causes electrons to be removed from the atoms (it is ionised) leaving +1 positive ions in the chamber.
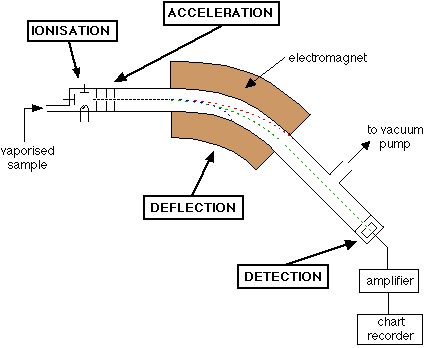
Describe the process of acceleration that occurs in a mass spectrometer
The positively charged ions are attracted to the negatively charged plates in an electric field, causing them to accelerate.
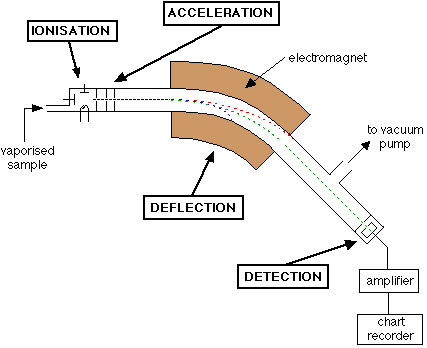
Describe the process of deflection that occurs in a mass spectrometer
The ions are deflected by a magnetic field.
The amount of deflection depends on the mass and charge of the ion. (lighter ions are deflected more than the heavier ones)
What is fragmentation?
some molecular ions break down into smaller fragments (involves the breaking of covalent bonds between the atoms in the molecule), which are the other peaks on a mass spectrum, breaks into a positive fragment ion and a neutral fragment.
Examples:
CH3COOH(g) + e− → CH3COOH+ (g) + 2e−
M(g) + e− → M+(g) + 2e−
How is a fragmentation pattern produced?
the cation fragments are detected in the mass spectrometer
How to determine the mass of neutral fragment lost?
draw its structure as well
m/z of molecular ion − m/z of cation fragment =
mass of neutral fragment lost
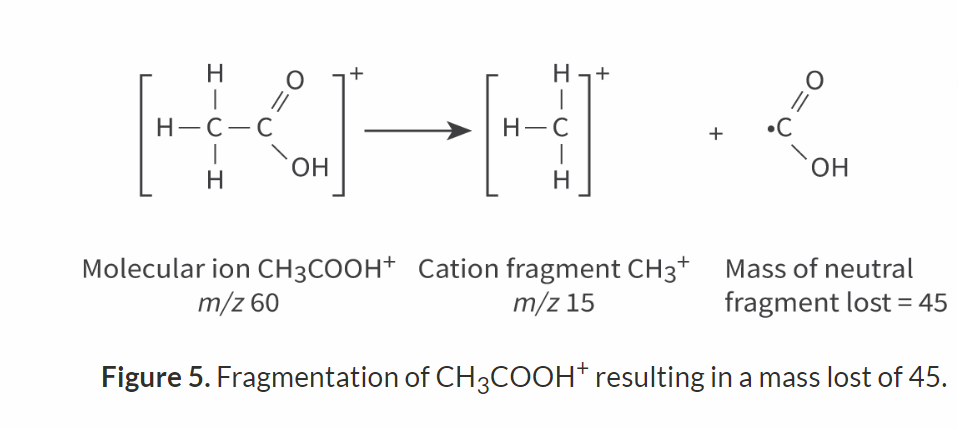
For the peak at m/z 15, this would be:
mass of molecular ion (60) − mass of cation fragment (15) = mass of neutral fragment lost (45)
What is infrared spectroscopy?
An analytical technique that uses IR radiation to determine the type of bonds present in an organic compound.
How do chemical bonds behave? (IR )
Rather than being rigid, they vibrate at specific frequencies.
If they are exposed to electromagnetic radiation of the same frequency at which the bond vibrates, the bond will absorb the radiation and vibrate
Different bonds absorb different amounts of infrared radiation
What are the two modes of vibration? (IR)
Stretching:
Symmetric and asymmetric
Bending
What are the two main regions of an infrared spectrum?
Fingerprint region (400-150):
determine the identity of an organic compound
Functional group region(from1500 upwards):
determine the types of bond present in the molecule
IR absorption of CO2 and H2O
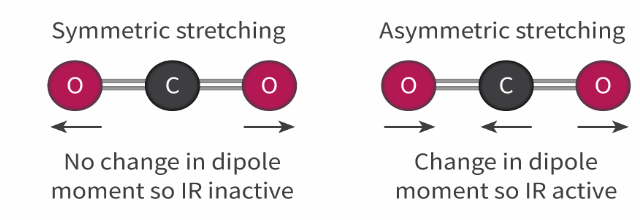
a molecule does not have to have a permanent dipole (such as CO2) but there must be a change in the dipole moment of the molecule
CO2 is a non-polar molecule because the bond dipoles cancel out. When the CO2 molecule undergoes symmetrical stretching, there is no change in the dipole moment of the molecule, therefore it does not absorb IR radiation and is known as being IR inactive
However, when the CO2 molecule undergoes asymmetric stretching there is a change in the dipole moment of the molecule because the bond dipoles no longer cancel out. This change in the dipole moment means that the CO2 can now absorb IR radiation and is known as being IR active
When is a molecule IR active?
When its a polar molecule in all three states
When its non-polar but asymmetric stretch due to dipole-dipole movement
How does bond angle relate to global warming? (IR spec)
The greenhouse gas effect is dependent on its:
Atmospheric condition
ability to absorb infrared radiation
Some o the shorter wavelength incoming radiation (Visible and UV) is reflected back into space and some are absorbed by the atmosphere before it reaches earths surface.
The energy reflected back from the earth’s surface is longer wavelength infared radiation.
Greenhouse gases such as water and CO2 allow the passage of incoming shortwave radiation, but absorb some of the longer wave reflected infrared radiation and radiate back into the earth’s surface, which increases the earths temperature.
What does HNMR help identify?
Proton nuclear magnetic resonance (H NMR) spectroscopy helps to determine the number of different chemical environments of hydrogen atoms in a molecule.
an example of spin resonance spectroscopy.
How does HNMR work?
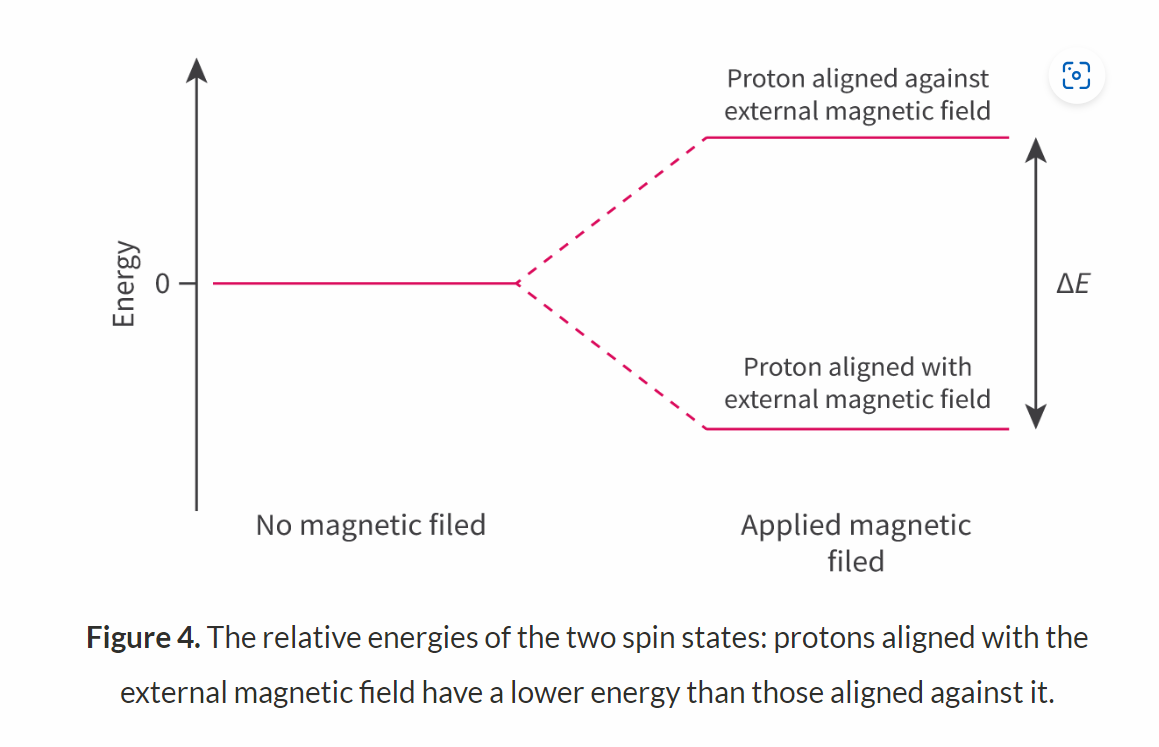
The nuclei of hydrogen atom posses spin and can exist in two possible states of equal energy.
In particles with odd numbers of nucleons, such as 1H, the spins do not cancel out.
IF a strong magnetic field is applied:
the spin states may align themselves either with the magnetic field or against it
there is a small energy difference between these alignments (can be provided by radio waves).
If the protons aligned with the magnetic field are exposed to resonance frequency
some of these protons flip from the lower energy state to the higher energy state.
When the radio frequency signal is switched off, these protons will return to the lower energy state and re-emit the same frequency of radiation that was absorbed.
This radiation is then detected by the 1H NMR spectrometer producing a 1H NMR spectrum
What’s a chemical shift?
A measure of the frequency of radiation absorbed molecule relative to TMS.
How are the bond peaks measured in HNMR?
It is measured against a standard molecule tetramethylsilane .
Standard molecule as 4 identical hydrogen and carbon environments.
It is unreactive and non-toxic.
It is volatile
has a low boiling point. it can easily be removed from the sample being analysed.
All the protons in TMS are equivalent (in the same chemical environment), therefore, it produces a strong single signal (or peak)
upfield
, away from other signals.
Chemical environment
A group of equivalent protons that all have the same resonance frequency. Also known as the hydrogen environment.
Protons in the same chemical environment are known as equivalent protons and those in different chemical environments are known as non-equivalent protons.
What do the different number of peaks represent?
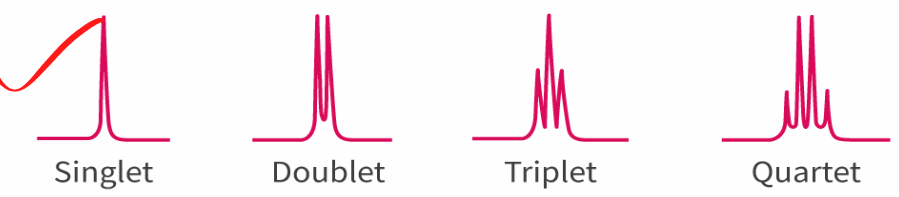
Singlet: 0 H on adjacent carbon
Doublet: 1 H on adjacent carbon/ 1:1
Triplet: 2 H on adjacent atom /1:2:1
Quartet: 3 H on adjacent carbon /1:3:3:1
follows a n+1 rule where n is the number of hydrogen on the adjacent carbon.
What can a 1H NMR spectrum be used to determine?
The number of different chemical environments that contain protons, which is shown by the number of signals on the spectrum.
The type of protons in each different chemical environment, which is shown by the
chemical shift
of the signal.
The ratio of protons in each different chemical environment, which is shown by the
integration trace
The number of adjacent hydrogens is shown by splitting patterns
What results in the splitting pattern?
When observing a high resolution 1H NMR spectrum we see that the peaks produced by the different chemical environments are split into clusters of peaks (multiplets), which are known as splitting patterns
. This is a result of the interactions between the magnetic fields of adjacent non-equivalent protons, known as
spin–spin coupling
.
What does the number of peak in an HNMR suggest?
indicates the number of different hydrogen bonds present in the molecule
What does a chemical shift in an HNMR suggest?
gives information about the electron density of the specific hydrogen element
What does the ratio of peak areas in an HNMR suggest?
Number of protons that each peak uses can be used to determine the different types of protons in the molecule
What does the splitting pattern in an HNMR suggest?
amount of carbons in an adjacent atom
How is energy supplied in the different types of spectroscopy?
radio waves for HNMR (intrinsic spin properties of atomic nuclei.)
infared waves for IR
mass spectrometry, the energy is supplied by bombarding the sample with high-energy electrons.