Energetics, Enzymes, and Metabolic Pathways in Biology
1/94
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
95 Terms
Kinetic energy
Energy that an object possesses due to motion or movement.
Potential energy
Energy that is stored in an object or system as a result of position, shape, or state.
Free energy
A thermodynamic concept that refers to energy that is available to do work.
Activation energy
The minimum amount of energy that is needed for a chemical reaction to occur.
Exergonic reactions
Reactions that are spontaneous.
Endergonic reactions
Reactions that are nonspontaneous.
Thermodynamics
The study of energy, its transformation, and its relationship to work and heat.
Enthalpy
Total energy of a system.
Metabolism
The sum total of chemical reactions that occur within a living organism to maintain life.
Catabolism
The process that breaks down complex molecules into simpler ones, releasing energy.
Anabolism
The process that uses energy to synthesize complex molecules from simpler ones.
Coupled reactions
Two chemical reactions are said to be coupled when the energy released from one reaction is used to drive the other reaction.
Phosphorylation
The addition of a phosphate group increases the potential energy of a molecule by adding negative charge and causing changes in conformation.
First Law of Thermodynamics
Energy cannot be created or destroyed, but it can change form.
ATP
Adenosine triphosphate is the molecule that serves as the primary energy currency of the cell.
Second Law of Thermodynamics
In any isolated system, the total entropy (disorder) always increases over time.
Bioenergetics
The study of energy flows through a living system.
Chemical energy
Energy stored in chemical bonds (potential); energy released (kinetic).
Chemical reactions
The making and breaking of bonds between atoms.
Transition state
The unstable state that reactants reach when they are contorted and unstable, allowing the bond(s) to be broken or made.
Activation Energy (in context)
The energy required for a reaction to proceed, causing reactant(s) to become contorted and unstable.
Chemical Reaction
X + A-B X-A + B
Free Energy Formula
Energy of reactants - Energy of the products = change in energy (ΔG)
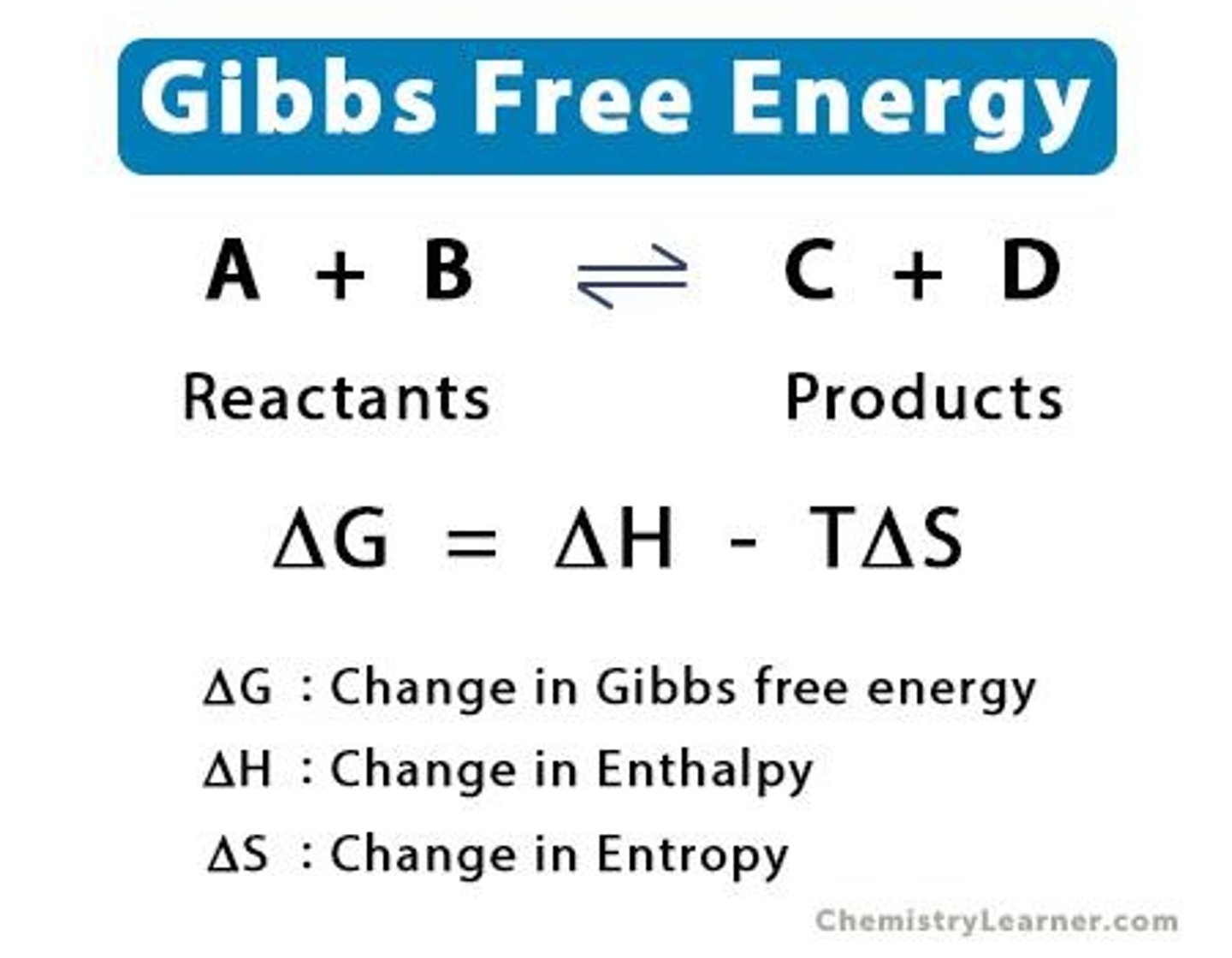
Exergonic Reaction
A reaction where ΔG is negative.
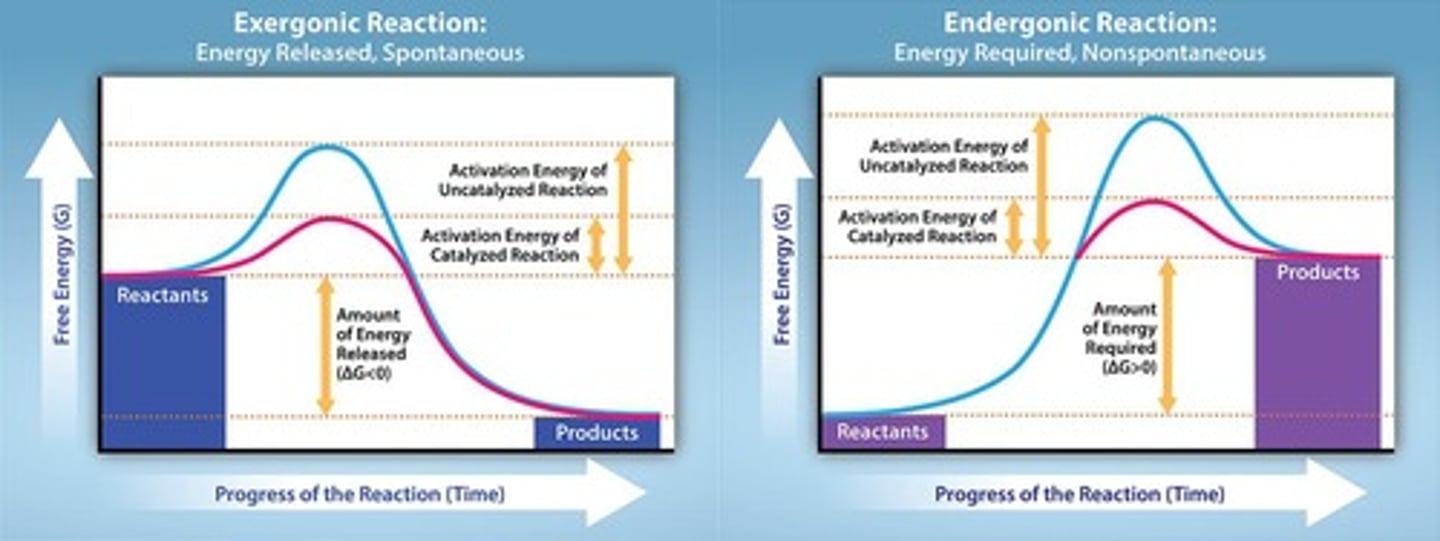
Endergonic Reaction
A reaction where ΔG is positive.
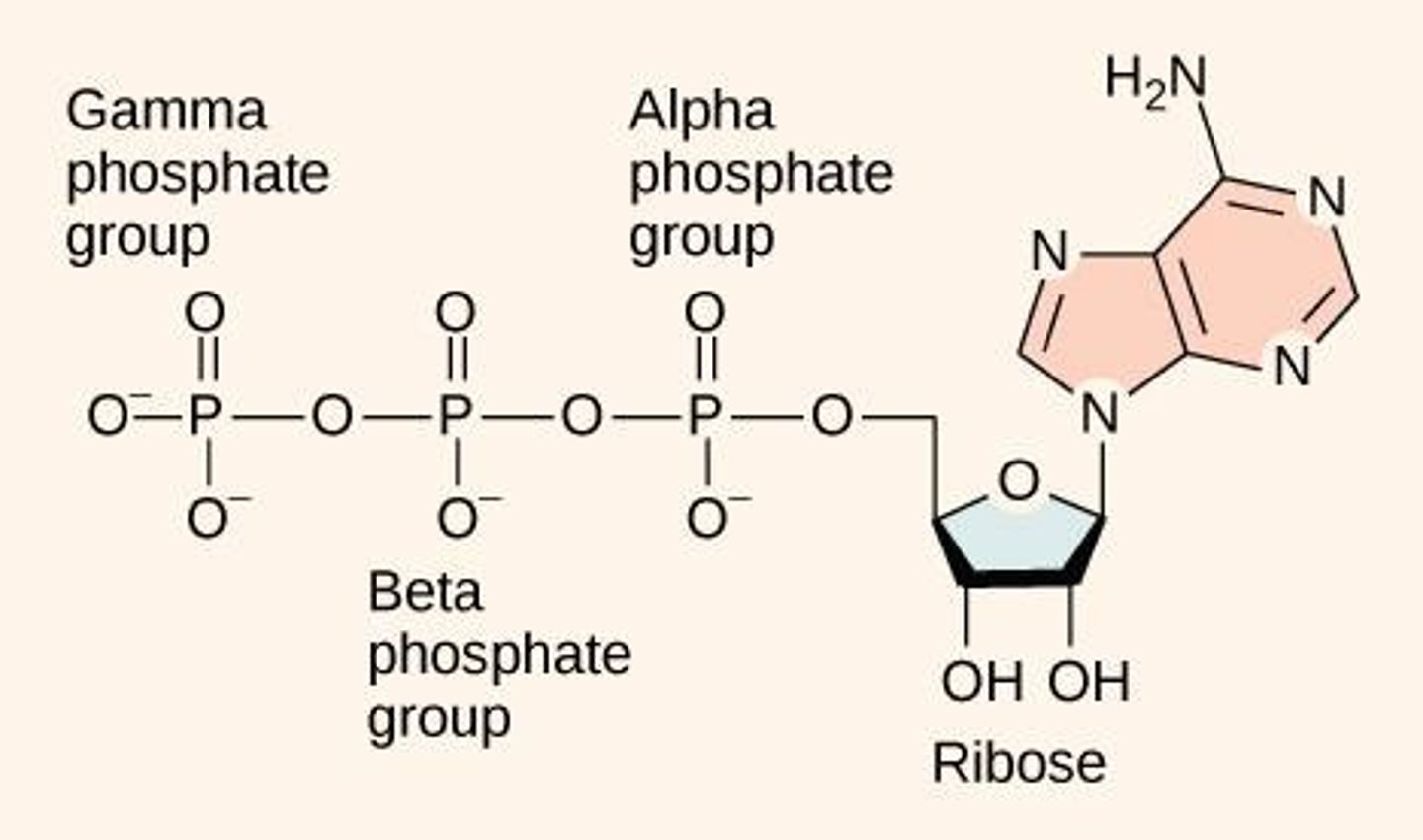
ATP Structure
Composed of an adenosine backbone with three phosphate groups attached.
High-Energy Bonds
The bonds that link the phosphate groups in ATP; breaking them releases energy.
ATP Hydrolysis
ATP → ADP + Pi + Energy; ΔG = -7.3 kcal/mol.
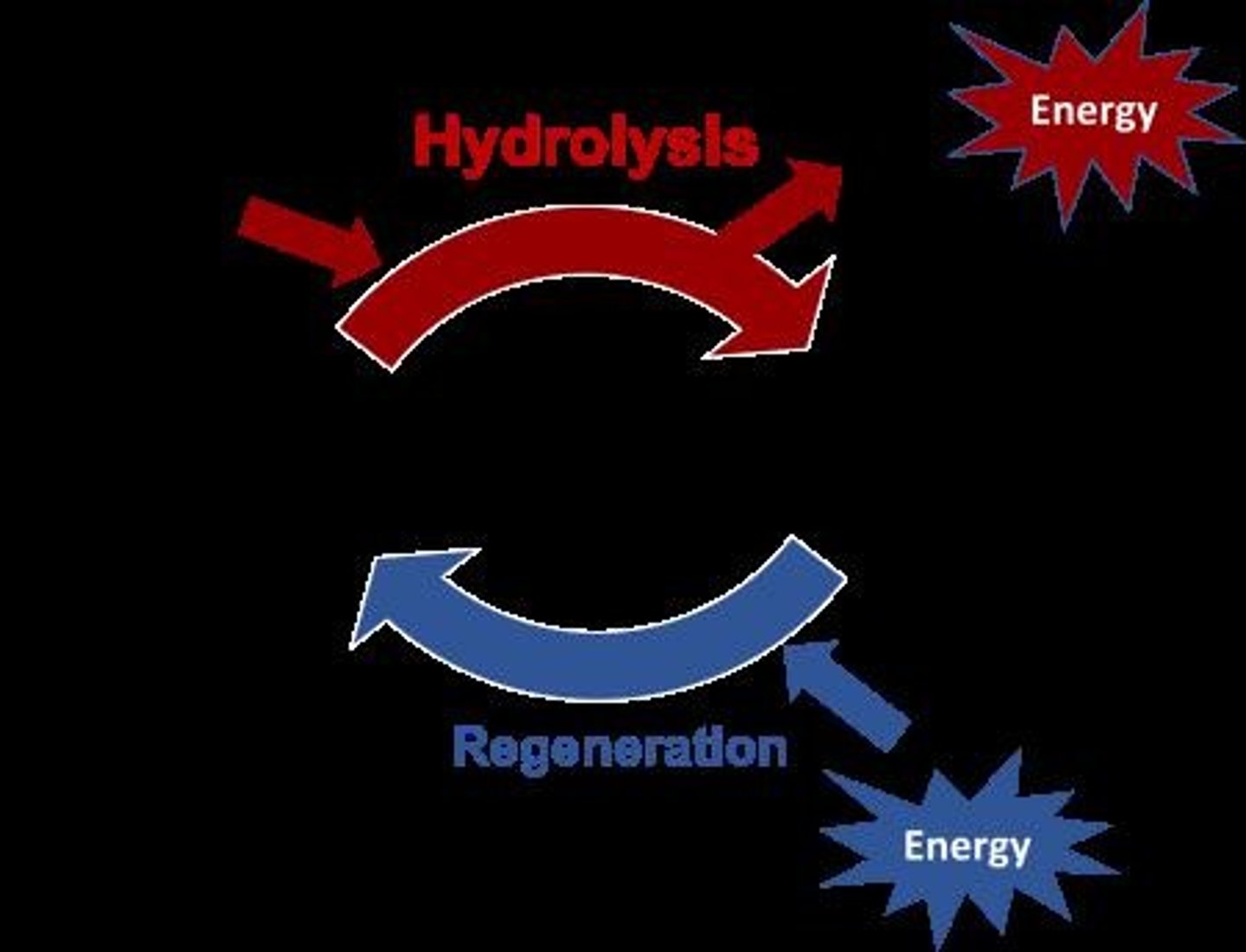
ATP Coupling
ATP → ADP + Pi + Energy; A + B + Energy → A-B.
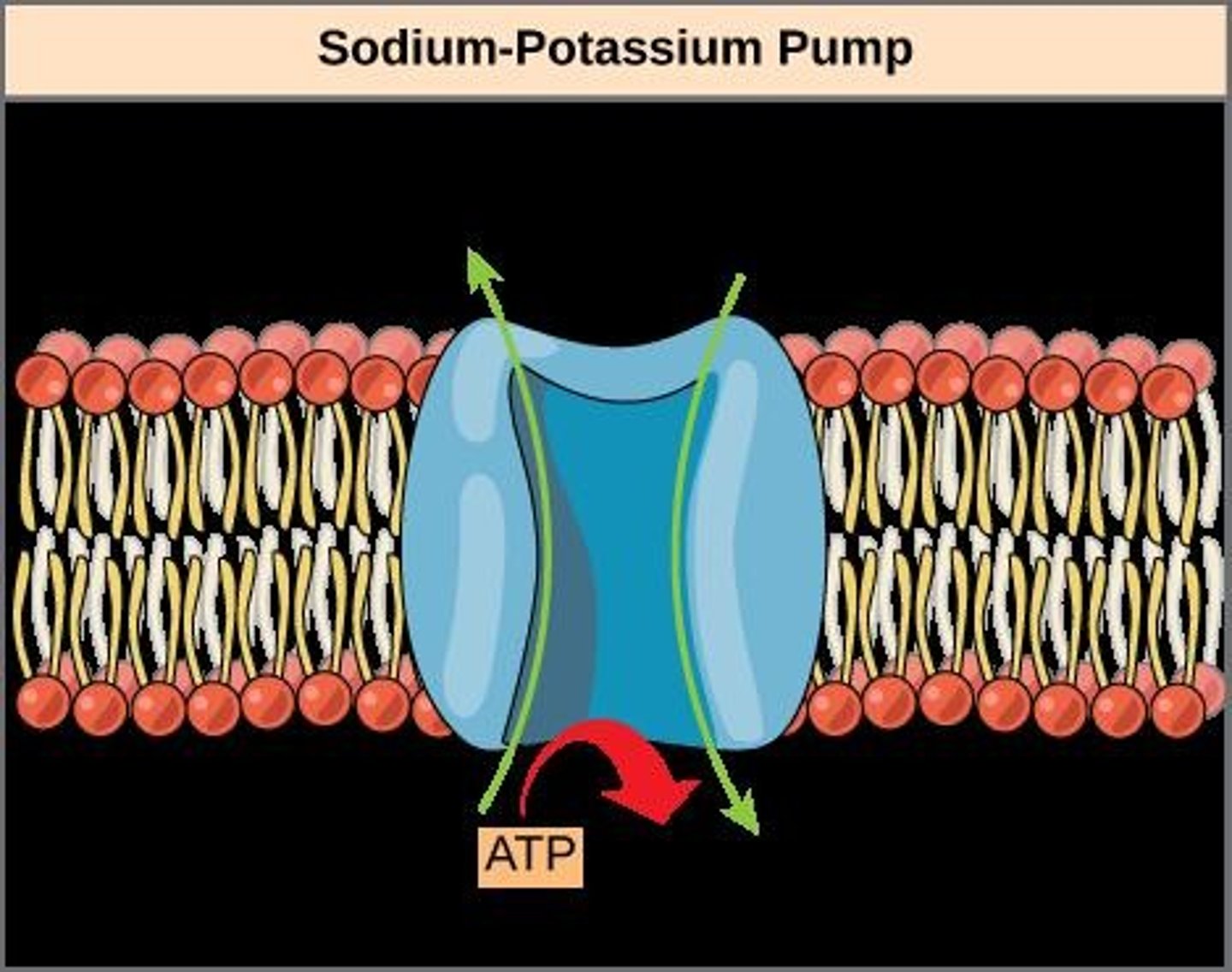
Sodium-Potassium Pump
An example of energy coupling using ATP hydrolysis to pump 3 sodium ions out and 2 potassium ions into the cell.
Metabolic Pathway
A series of biochemical reactions that converts one or more substrates into a final product.
Anabolic Pathways
Reactions that require energy and synthesize larger molecules.
Catabolic Pathways
Reactions that release energy and break down large molecules into smaller molecules.
Dehydration Synthesis
The process of linking two glucose molecules to form the disaccharide maltose, releasing a water molecule.
Hydrolysis
The process of breaking down polymers into individual monomers, using water as a reactant.
Maltose Hydrolysis
The disaccharide maltose is broken down to form two glucose monomers.
Enzymes
Proteins and some RNA molecules that act as biological catalysts. They accelerate chemical reactions by lowering the activation energy, thereby speeding up the rate of a specific chemical reaction without being consumed or altered in the process.
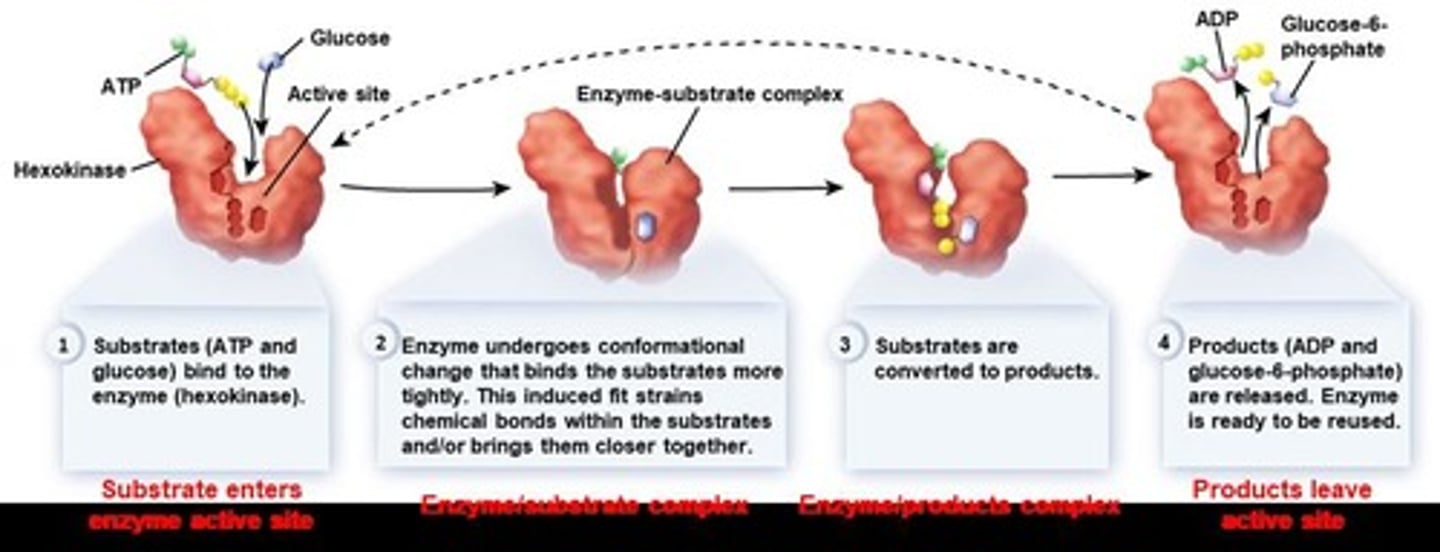
Active site
The specific region on an enzyme where the reaction occurs. Enzyme is 'actively' involved in the reaction at this site.
Specificity
The active site is shaped to fit a particular substrate molecule, and only that substrate molecule will be able to bind to it with high affinity.
Reaction rate
Enzymes increase reaction rate by lowering activation energy.
Optimal range
The specific range of conditions (such as pH and temperature) at which an enzyme exhibits its maximum catalytic activity.
Denaturation
A process in which the 3D structure of an enzyme is altered in such a way that it can no longer perform its catalytic function.
Saturation point
Stage at which all enzyme active sites are occupied by substrate molecules. Increasing [substrate] further does not increase reaction rate.
Feedback Inhibition
A regulatory mechanism in which the end product of a metabolic pathway inhibits an enzyme involved in its synthesis.
Competitive Inhibition
A form of enzyme inhibition where the inhibitor competes with the substrate for binding to the active site.
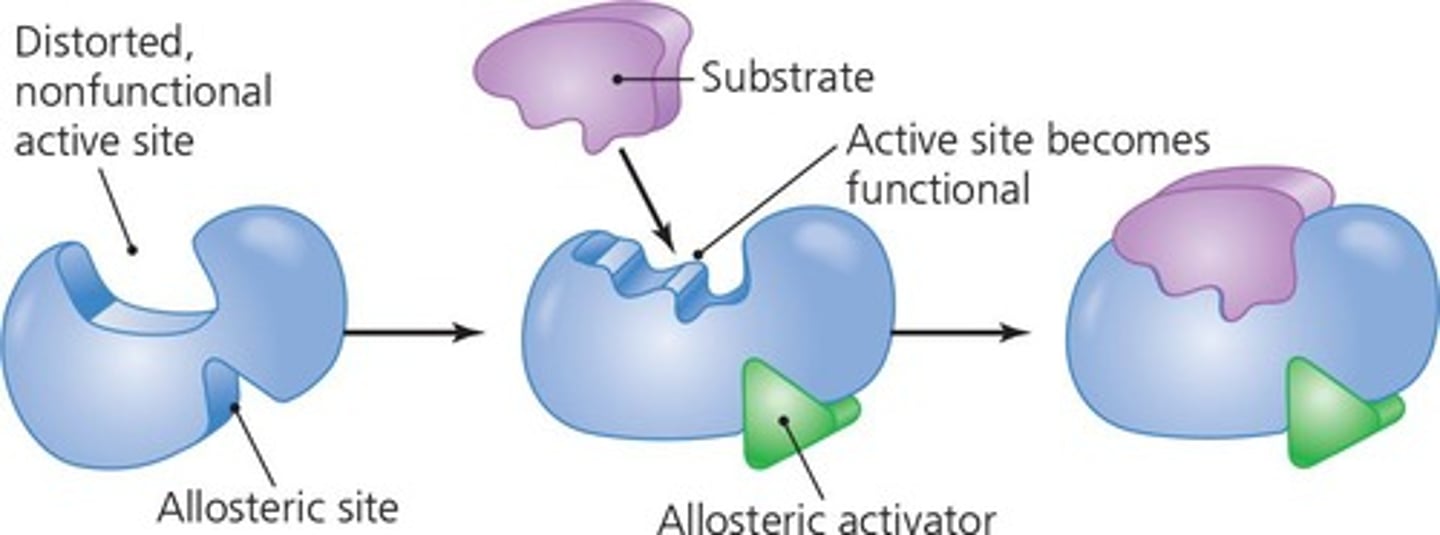
Noncompetitive (allosteric) Inhibition
A form of enzyme inhibition where the inhibitor binds to an allosteric site, changing the enzyme's shape and function.
Allosteric activation
A process where an effector molecule binds to an allosteric site on an enzyme, enhancing its activity.
pH effect on enzyme reactions
The activity of enzymes can be significantly affected by the pH level of the environment, with each enzyme having an optimal pH range.
Temperature effect on enzyme reactions
Enzymes have an optimal temperature at which they function most efficiently, and deviations can lead to decreased activity or denaturation.
Substrate concentration effect on enzyme reactions
Increasing substrate concentration can enhance the reaction rate up to a saturation point, beyond which the rate levels off.
Hexokinase
An example of an enzyme that has an optimal temperature of 37°C and pH 7.4, suggesting it functions in the human body.
Enzyme specificity
Most enzymes catalyze one specific reaction due to the precise fit between the enzyme's active site and its substrate.
Enzymes as catalysts
Enzymes are protein catalysts that speed up reactions by lowering the required activation energy.
Bond-breaking and bond-forming processes
Enzymes bind with reactant molecules, promoting bond-breaking and bond-forming processes.
Ribozymes
Non-protein enzymes that exist, which are RNA molecules capable of catalyzing chemical reactions.
Enzyme-Substrate Specificity
The 3D shape of the enzyme and reactants (aka substrates) determines this specificity.
Catalyze
Enzymes can catalyze a variety of reactions, including bonding two substrates together or breaking down one molecule into smaller products.
Lysozyme
An enzyme found in various biological systems, including tears, saliva, mucus, and egg whites, that breaks down bacterial cell walls by hydrolyzing glycosidic bonds in peptidoglycan.
Peptidoglycan
A major component of the bacterial cell wall that is hydrolyzed by lysozyme.
Induced Fit
A mild shift in shape at the active site that optimizes reactions, maximizing enzyme activity.
Lock-and-Key Model
A previously held model of enzyme-substrate interaction, which has been expanded by the induced fit model.
Glycolysis
The metabolic pathway that converts glucose into pyruvate, with hexokinase catalyzing the first step.
Glycogen Synthesis
The process of forming glycogen from glucose, with hexokinase catalyzing the first step.
Conformational Changes
The changes that occur in the active site of an enzyme upon substrate binding, leading to a more precise fit.
Biochemical Reaction Speed
The speed of the reaction is increased by the dynamic binding of the enzyme to the substrate.
Protein Structure
The 3-D shape of a protein is determined by the amino acid sequence of the polypeptide.
Amino Acids of the Active Site
Particularly important for the enzyme's function, allowing binding with unique substrate(s).
ATP (Adenosine Triphosphate)
The molecule that provides energy for the phosphorylation of glucose by hexokinase.
Substrate
The reactant molecule that binds to the enzyme's active site.
Binding Sites
Regions on the enzyme where substrates can attach.
Catalytic Site
The specific area of the enzyme where the chemical reaction occurs.
Denature
The process in which an enzyme loses its functional shape due to suboptimal temperatures.
Substrate-Enzyme Binding
The interaction between a substrate and an enzyme that can be reduced by suboptimal pH.
Enzyme Mechanisms
Ways in which enzymes lower activation energy, including positioning substrates, providing optimal environments, contorting substrates, and temporarily reacting with substrates.
Metabolic Control
The regulation of enzyme activity to maintain metabolic homeostasis and respond to environmental changes.
Energy Conservation
The purpose of enzyme regulation that prevents unnecessary consumption of resources.
Response to Environmental Changes
The ability of organisms to adapt by upregulating or downregulating specific enzymes based on varying conditions.
Coordination of Cellular Processes
The function of enzymes as components of complex signaling networks to integrate signals and respond accordingly.
Enzyme Regulation
The control of enzyme activity through various mechanisms including temperature, pH, and the presence of inhibitors or activators.
Competitive Inhibitors
Molecules that slow reaction rates by competing with the substrate for the active site without affecting the maximal rate.
Noncompetitive Inhibitors
Molecules that slow reaction rates and reduce the maximal rate by binding to an enzyme at a site other than the active site.
Allosteric Inhibition
The regulation of an enzyme by binding an inhibitor at a site other than the active site, altering the enzyme's activity.
Cofactors
Non-protein molecules that assist enzymes in catalyzing reactions.
Coenzymes
Organic molecules that act as cofactors and are necessary for enzyme activity.
Optimal Environment
Conditions such as pH or temperature that are most favorable for enzyme activity.
Substrate Stability
The likelihood of a substrate to react, which can be influenced by enzyme interactions.
Enzyme Availability
The state of an enzyme being ready to catalyze a reaction after releasing the product.
Allosteric Inhibitors
Modify the active site of the enzyme so that substrate binding is reduced or prevented.
Allosteric Activators
Modify the active site of the enzyme so that the affinity for the substrate increases.
Noncompetitive Inhibition
Inhibition of enzyme activity at an allosteric site, not affecting substrate binding directly.
Metabolic Pathways
A series of biochemical reactions catalyzed by multiple enzymes.
Isoleucine
Acts as an allosteric inhibitor of the isoleucine synthesis pathway.
Enzyme Cofactors
Some enzymes require one or more cofactors or coenzymes to function.
Optimal Temperature
The temperature at which an enzyme, such as hexokinase, exhibits maximum activity, e.g., 37°C.
Optimal pH
The pH at which an enzyme exhibits maximum activity, e.g., pH 7.4.