Equilibrium Constant Kc & Kp
0.0(0)
Card Sorting
1/7
Earn XP
Description and Tags
Last updated 9:06 AM on 7/9/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
1
New cards
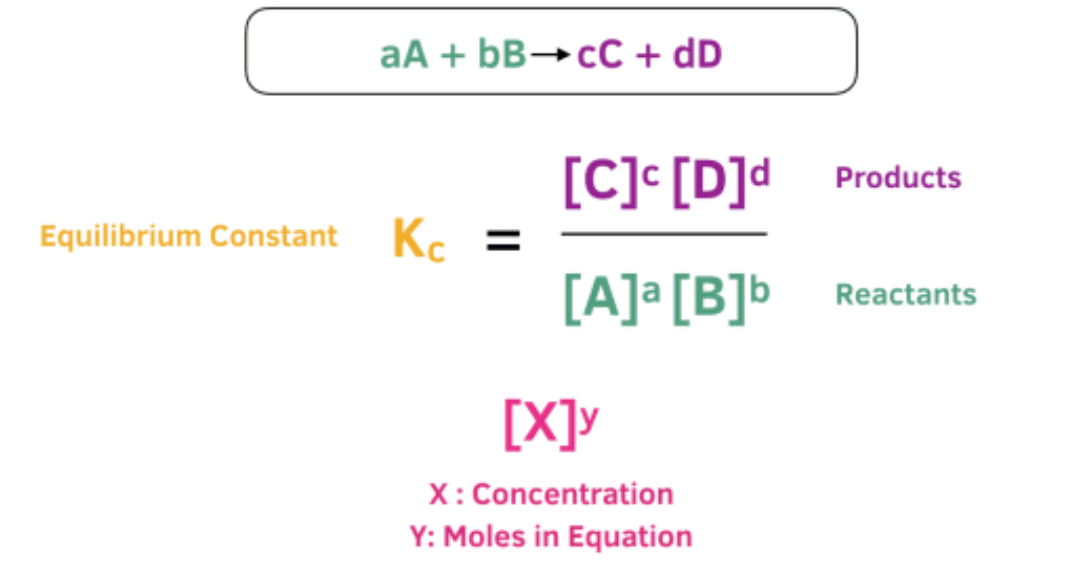
What is Kc
The equilibrium constant (K) is a mathematical expression that describes the ratio of product to reactant concentrations at equilibrium in a chemical reaction
2
New cards
When is Kc only used
In a homogeneous reaction- with reactants and products in the same phase. For heterogeneous reactions, the concentration of the solid is ignored because it is dependent on its density rather than volume
3
New cards
Why is a high value of Kc/Kp preferred
Because it favours the production of products
4
New cards
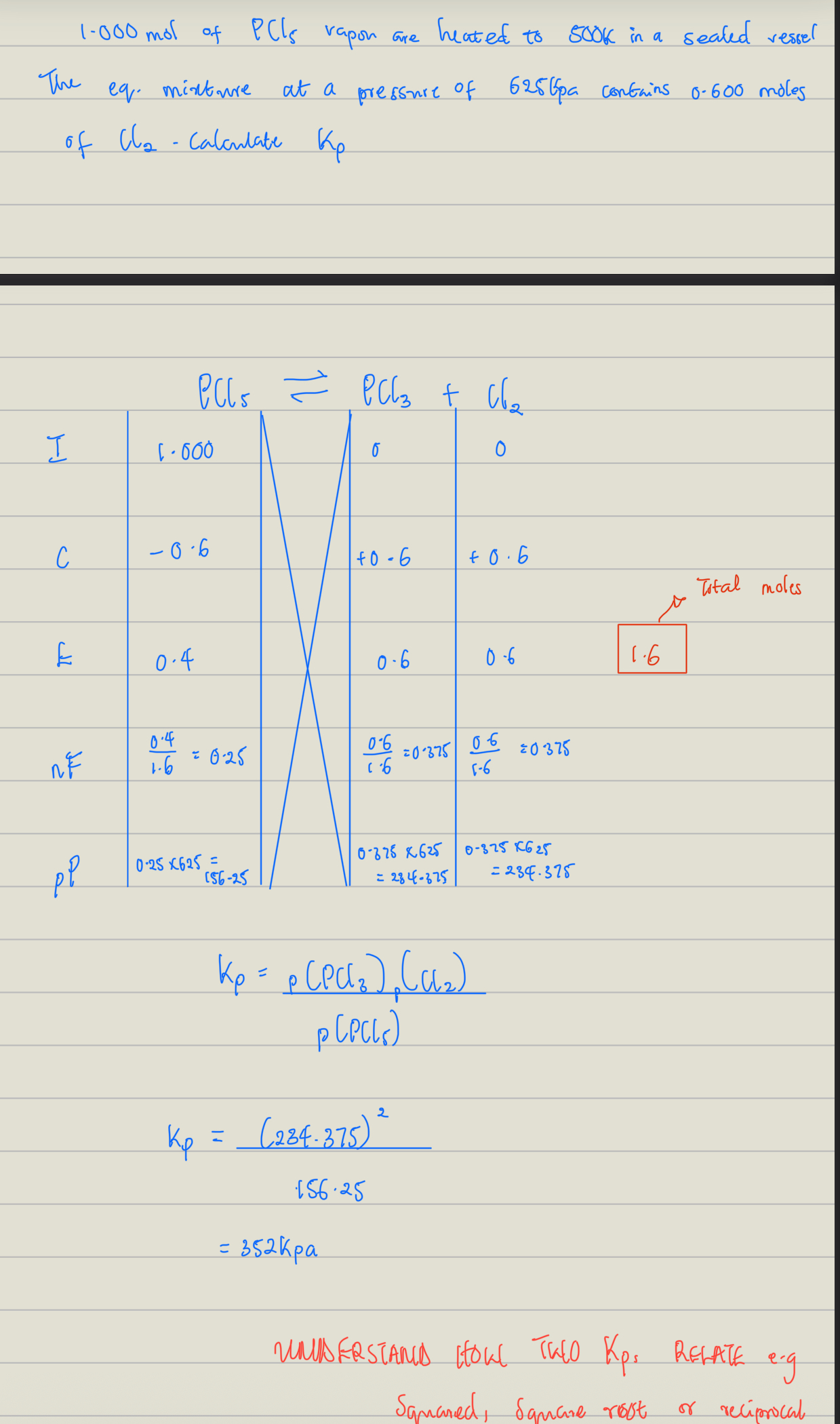
Example of Calculations

5
New cards
What factors affect Kc/Kp
Only temperature affects Kc
6
New cards
When is Kp used
With gases
7
New cards
How is partial pressure calculated

8
New cards
Example of calculation