Lecture 16 - lipids
what are Glycosphingolipids
subclass of sphingolipids
Located on the outer face of plasma membranes important in cell surface recognition events.
two types
1. Cerebrosides
2. Globosides
characteristics of Globosides
Head group = 2 or more sugar units (typically 3–9)
Still uncharged overall
Found in various cell types, especially in red blood cells
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
61 Terms
what are Glycosphingolipids
subclass of sphingolipids
Located on the outer face of plasma membranes important in cell surface recognition events.
two types
1. Cerebrosides
2. Globosides
characteristics of Globosides
Head group = 2 or more sugar units (typically 3–9)
Still uncharged overall
Found in various cell types, especially in red blood cells
characteristics of Cerebrosides
Head group = 1 sugar unit (e.g., glucose or galactose)
No net charge
Found in neuronal cell membranes
what are human blood group antigens like (O,A,B)
Are glycosphingolipids
Differ only in their carbohydrate head groups
Type O = basic structure
Type A and B = addition of specific sugar(s)
what are Gangliosides
Sphingolipids with:
A ceramide backbone
An oligosaccharide head group
At least one sialic acid residue (N-acetylneuraminic acid, or Neu5Ac)
Key Features:
Negatively charged at physiological pH (pH 7) due to the sialic acid.
This makes them different from globosides, which also have multiple sugars but are neutral.
Function and Importance:
Found in neuronal cell membranes, especially in the brain.
Involved in:
Cell-cell recognition
Signal transduction
Immune response
what are two lipids that are not derived from fatty acids?
(No Fatty Acids)
Examples:
Steroids (e.g. cholesterol, testosterone)
Terpenes (e.g. vitamins A, E, K, and plant pigments like carotenoids)
what are steroids and tepenes synthesized from
Both steroids and terpenes are derived from isoprene units.
The activated form is called isopentenyl pyrophosphate (IPP).
how are terpenes built from isoprene units
Terpenes are formed as multiples of isoprene units joined mainly head to tail and occasionally tail to tail:
Classification of terpene by Size
Terpene Type | # of Isoprene Units | Carbon Count | Example |
|---|---|---|---|
Monoterpene | 2 | C₁₀ | Geraniol |
Sesquiterpene | 3 | C₁₅ | Farnesene |
Diterpene | 4 | C₂₀ | Retinol (Vitamin A) |
Triterpene | 6 | C₃₀ | Squalene |
Tetraterpene | 8 | C₄₀ | β-carotene |
what is B-Carotene
. a tetraterpene pigment: Each 5-carbon isoprene is indicated in a different colour.
is a precursor to Vitamin A (retinol/retinal)
how does B-Carotene become retinol (vitamin A1)
β-Carotene (a tetraterpene) is cleaved in the center by an enzyme called a dioxygenase.
This produces two molecules of all-trans-retinal.
Then, retinal reductase can convert retinal → all-trans-retinol (Vitamin A₁).
why is vitamin A important?
Vitamin A₁ (all-trans-retinol) is essential for vision.
It is later converted into 11-cis-retinal, which binds to opsin to form rhodopsin in the retina (more on this in the next slides).
Without sufficient β-carotene (or Vitamin A), vision can be impaired, especially in low light (night blindness).
Carrots, rich in β-carotene
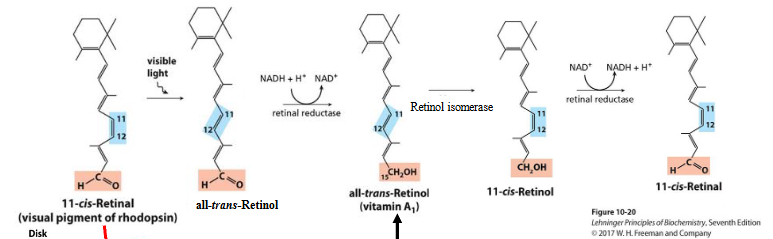
what happens to all trans retinol?
All trans retinol is isomerized to 11-cis retinol and then oxidized to 11-cis retinal
Step-by-Step Conversion: of above
All-trans-Retinol (Vitamin A₁):
Derived from β-carotene
Contains an alcohol group (–CH₂OH) at the end
Isomerization:
Enzyme: Retinol isomerase
Converts all-trans-retinol into 11-cis-retinol
Key difference: rotation around C11=C12 double bond to form cis configuration
Oxidation:
Enzyme: Retinal dehydrogenase (retinal reductase in reverse)
Converts 11-cis-retinol into 11-cis-retinal
Functional group changes: –OH → =O
Why 11-cis-Retinal Matters:
11-cis-retinal is the light-sensitive chromophore that binds to opsin to form rhodopsin in rod cells of the retina.
It plays a key role in initiating visual signal transduction upon light exposure.
what is rhodopsin?
the light-sensitive complex that initiates visual signaling.
how is it formed
11-cis-retinal binds to a membrane protein called opsin.
Together they form the complex known as rhodopsin.
Rhodopsin is located in the disk membranes of rod cells in the retina.
What Makes Rhodopsin Special?
11-cis-retinal acts as a chromophore (light-absorbing group).
Different opsin proteins (due to amino acid sequence variations) adjust the light absorption properties of 11-cis-retinal.
This allows detection of different wavelengths of light:
Blue light: λₘₐₓ = 440 nm
Green light: λₘₐₓ = 530 nm
Yellow light: λₘₐₓ = 570 nm
Rhodopsin Function Summary:
It's the primary photoreceptor in rod cells.
Absorbs light, initiating a conformational change (shown in the next slides).
Triggers a G-protein–mediated signaling cascade leading to a nerve signal to the brain.
what happens when visible light hits rhodopsin?
At rest (in the dark):
We have rhodopsin, which is opsin bound to 11-cis-retinal.
This complex is ready to detect light, but nothing is "removed" yet.
Rhodopsin is inactive but primed — like a loaded spring waiting for a trigger.
When light hits rhodopsin:
11-cis-retinal is isomerized into all-trans-retinal — it changes shape.
What Happens Next? (4)
Rhodopsin changes conformation due to retinal’s shape change.
This activates a G-protein called transducin.
Transducin triggers a signaling cascade, which includes:
Opening of Ca²⁺ channels
Calcium influx into the rod cell
This leads to nerve excitation, sending the vision signal to the brain.
what is the first detectable event in vision?
The first detectable event in vision is the photoisomerization of retinal.
This is a fast and highly sensitive process — even a single photon can trigger it!
what happens after photoisomerization
11-cis-retinal becomes all-trans-retinal.
All-trans-retinal is no longer a good fit for opsin, so it:
Detaches from rhodopsin
Diffuses away from the membrane
what happens to all trans retinal at the end?
The all-trans-retinal is reduced back to all trans retinol after which it can undergo the process of conversion back to 11-cis-retinal

Visual Cycle Overview
11-cis-retinal binds to opsin → forms rhodopsin
Light hits rhodopsin → photoisomerization of 11-cis-retinal to all-trans-retinal
This causes:
Rhodopsin conformational change
Activation of transducin
Nerve signal to the brain
All-trans-retinal detaches from opsin and is recycled:
Recycling Steps:
Reductase:
Converts all-trans-retinal → all-trans-retinol
(reduction step)
Isomerase:
Converts all-trans-retinol → 11-cis-retinol
(rearranges geometry)
Reductase again:
Converts 11-cis-retinol → 11-cis-retinal
(oxidation step to restore aldehyde group)
what is Cholecalciferol and how is formed?
is Vitamin D₃
Where it comes from:
Formed in skin by an UV-induced photochemical reaction on 7-dehydrocholesterol:
7-dehydrocholesterol absorbs the UVB light
It is photochemically converted into previtamin D₃
function of d3
Acts as a hormone, not just a vitamin
Increases Ca²⁺ absorption in:
Intestines
Kidneys
Bone
Maintains calcium and phosphate balance, which is essential for bone strength
what disease happens if your Deficient in vit d3?
Causes rickets (soft/bowed bones in children)
Historically common in cold, low-sunlight areas due to:
Heavy clothing
Limited UV exposure during winter
what is Vitamin E?
is the collective name for a group of closely related lipids called tocopherols, all of which contain a substituted aromatic ring and a long isoprenoid side chai
Structure of vit e
Contains:
A substituted aromatic ring (chromanol ring)
A long isoprenoid side chain (shown in colored zigzags)
The ring enables redox reactions
The isoprenoid chain allows embedding in lipid membranes
functions of vit e
Acts as a biological antioxidant
Neutralizes oxygen radicals
Prevents non-enzymatic oxidative damage to:
Membrane lipids
Cell components
Protects membrane fluidity and integrity
Deficiency Symptoms:
Can lead to:
Sterility
Muscle weakness
Mechanism of these symptoms is still not fully understood
what is vit K?
Vitamin K is a family of lipids, differing in the unsaturated state of the chain attached to the napthoquinone:
naqthoquinone = A naphthoquinone is the chemical backbone of vitamin K — it's a two-ring structure with ketone groups, essential for its biological activity in clotting.
Structure:
Built on a naphthoquinone ring (aromatic + two ketone groups)
Has a long aliphatic side chain (highlighted with varying degrees of unsaturation in different forms)
Differences in side chain saturation define types of vitamin K (e.g., K₁, K₂, etc.)
function of vit K
Cofactor in blood clotting
Essential for the activation of prothrombin, a key blood-clotting protein
what does Deficiency in vit K cause?
Leads to impaired blood clotting
Can cause excessive bleeding, even from minor injuries
Newborns are especially at risk and are often given Vitamin K shots at birth
what is Coenzyme Q (Ubiquinone)?
Acts as an electron carrier in the electron transport chain (ETC) in mitochondria
Transfers electrons between complex I/II and complex III
structure of coenzyme Q
A quinone ring (oxidized form) with:
Two methoxy groups (–OCH₃)
A long isoprenoid tail (n = 4–8 isoprene units)
The isoprenoid tail makes it lipid-soluble, so it can diffuse within the inner mitochondrial membrane
what is Plastoquinone
Similar structure to ubiquinone but used in chloroplasts
Plays the same role: electron shuttle in the photosynthetic electron transport chain
Ubiquinone vs Plastoquinone
Molecule | Function | Location |
|---|---|---|
Ubiquinone | Electron carrier in respiration | Mitochondria |
Plastoquinone | Electron carrier in photosynthesis | Chloroplasts |
Both share a lipophilic isoprenoid chain and a redox-active quinone ring, making them mobile hydrophobic electron carriers in membrane-bound systems.
what are steroids?
a major class of isoprene-derived lipids with a distinctive four-ring structure known as the steroid nucleus.
structure of steroids?
Composed of 4 fused rings:
Three 6-carbon rings (A, B, C)
One 5-carbon ring (D)
Called the steroid nucleus (shown in diagram)
Planar and rigid, which influences how they interact with membranes and receptors
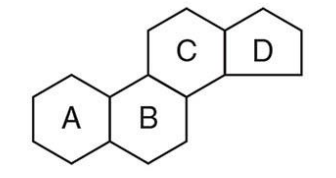
what are types of steroids?
Included in this family are the sex hormones, hormones secreted by the adrenal cortex, bile salts, and some poisons
what are non-structural steroids?
Hormones that regulate gene expression by binding to intracellular receptors.
Examples of Non-Structural Steroids:
Testosterone
Estradiol
Progesterone
Cortisol
Aldosterone
They are not structural membrane components like cholesterol, but instead act as signaling molecules.
Mechanism of Action:
Steroid hormones are lipid-soluble, so they can diffuse through membranes.
Inside the cell, they bind to nuclear receptors.
These hormone-receptor complexes then modulate transcription of specific genes
Testosterone vs Estradiol

Test vs Estradiol structure
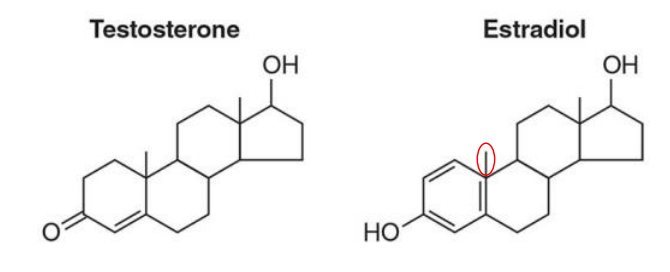
what is the most abundant steroid in the human body?
cholesterol
structure of cholesterol?
Based on the steroid nucleus (4 fused rings: A–B–C–D)
C3: Contains a hydroxyl group (–OH) → polar, forms the "head"
C17: Has an 8-carbon aliphatic chain → nonpolar, part of the "tail"
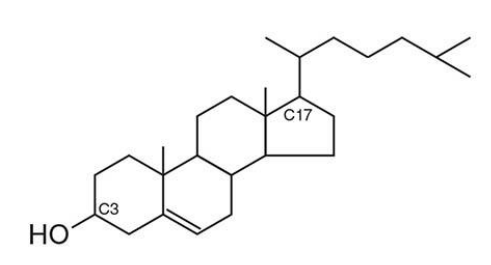
what does this structure orientation result in?
amphipathic molecule
Polar head: interacts with water/lipid headgroups
Nonpolar tail: embeds in the hydrophobic core of the membrane
what is cholesterols Function in Membranes:
Enriched in animal plasma membranes (~35% of total lipid content)
Regulates membrane fluidity:
Inserts between phospholipids
Stabilizes membranes at high temps
Prevents crystallization at low temps
How Cholesterol is Stored/Transported?
Cholesterol is esterified with fatty acids to form cholesteryl esters.
This:
Removes the –OH group at C3
Makes the molecule completely hydrophobic
Facilitates compact storage and transport in the body in lipoprotein complexes
what are lipoproteins?
Lipoproteins are spherical complexes that transport lipids (fats) like cholesterol, triglycerides, and fat-soluble vitamins through the aqueous bloodstream, which would otherwise repel these hydrophobic molecules.
structure of lipoproteins
Core:
Contains triacylglycerols and cholesteryl esters (both hydrophobic)
Surface:
Phospholipid monolayer (polar heads face out)
Free cholesterol and apolipoproteins (like ApoB-100) embedded in surface
These features help the lipoprotein stay soluble in the aqueous environment of blood.
why does density of lipoproteins matter?
Larger lipoproteins = lower density (more fat, less protein)
Smaller lipoproteins = higher density (less fat, more protein)
Types of Lipoproteins (in order of decreasing size & increasing density):
Type | Contents | Function |
|---|---|---|
Chylomicrons | Mostly dietary triglycerides | Transport lipids from intestine to tissues |
VLDL (Very Low Density Lipoprotein) | Triglycerides & some cholesterol | Made in liver, delivers lipids to tissues |
LDL (Low Density Lipoprotein) | Mostly cholesterol | Delivers cholesterol to cells → "bad cholesterol" |
HDL (High Density Lipoprotein) | Mostly protein, some cholesterol | Picks up excess cholesterol from tissues → returns to liver → "good cholesterol" |
which lipoproteins are healthy and not healthy?
High LDL = increased risk of atherosclerosis (plaque buildup)
High HDL = protective, helps clear cholesterol