To study the effect of concentration on reaction rate using sodium thiosulfate and hydrochloric acid
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
Theory
Sodium thiosulfate and hydrochloric acid react to produce a precipitate of sulfur as follows:
Na2S2O3 (Limiting) + 2HCl (excess) → 2NaCl + SO2 + S + H2O
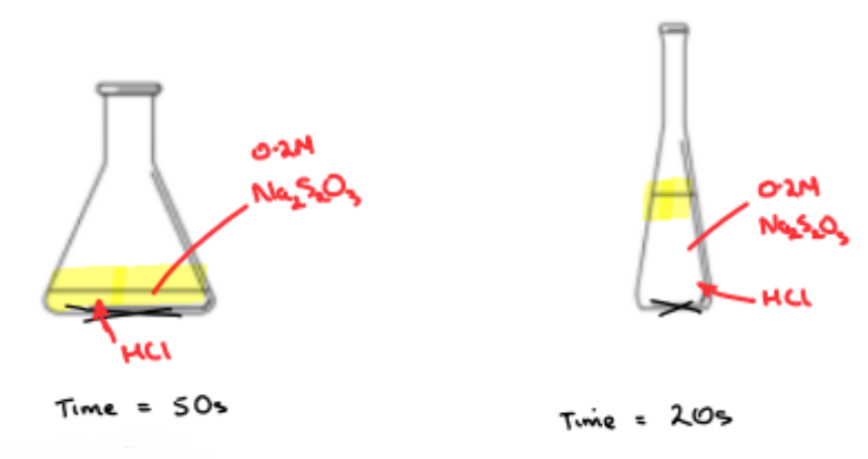
• Different concentrations of sodium thiosulfate are prepared and reacted against a dilute hydrochloric acid solution
• For each concentration, the time taken for a certain mass of the sulfur precipitate to form is recorded and the rate of reaction measured
Procedure
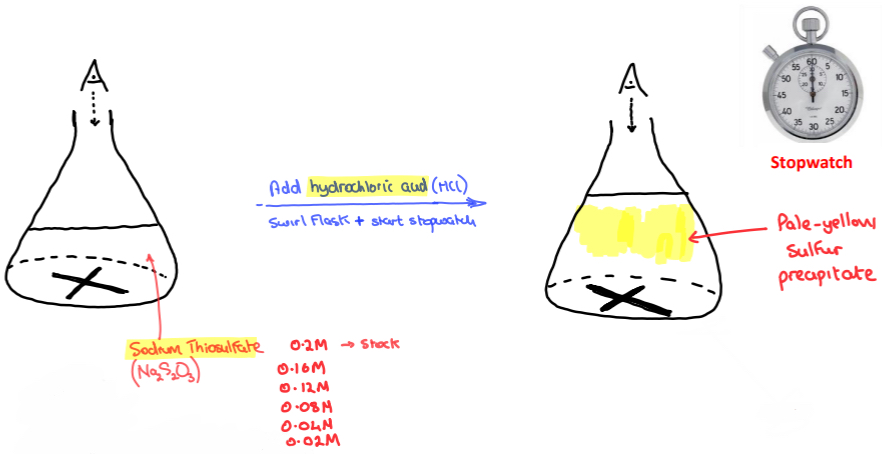
➢ Using a graduated cylinder, add a fixed volume of 0.2 M sodium thiosulfate is added to a conical flask
➢ The conical flask has a cross (‘X’) drawn on the bottom with a marker
➢ Using another graduated cylinder, a fixed volume of dilute hydrochloric acid is quickly added to the sodium thiosulfate solution in the conical flask
➢ The conical flask is swirled, and the stopwatch is immediately started
➢ While looking down through the solution, the time for it takes for the yellow sulfur precipitate to obscure the cross is recorded
➢ The experiment is repeated five times with DIFFERENT CONCENTRATIONS of sodium thiosulfate
➢ The results are entered in a table and a graph of rate (1/time) Vs concentration is plotted
Conclusion
A straight line graph through the origin is obtained showing rate of reaction is directly proportional to concentration of solution
Explain the relationship between rate of reaction and concentration of solution?
• Rate of reaction is directly proportional to concentration of solution
• A more concentrated solution has a greater number of particles
• A greater number of particles means greater frequency of collisions will occur
• Greater frequency of collisions means more effective collisions i.e more collisions reach the required activation energy
Why is it essential the same volume and concentration of hydrochloric acid are used during each reaction?
• Only one variable can be changed in order for the experiment to be a fair test i.e. the concentration of sodium thiosulfate
• All other possible variables must remain fixed
Identify the precipitate in this reaction? Describe its appearance
Sulfur – precipitates as a fine pale-yellow powder
Describe how the reaction time is measured in this experiment? / Describe how you could determine when the same mass of sulfur has been formed?
• The conical flask has an “X” drawn on the bottom
• The sodium thiosulfate and hydrochloric acid are mixed, and looking down through the solution, using a stopwatch, the time for how long it takes the yellow sulfur precipitate to obscure the cross is recorded
How is rate of reaction calculated?
Rate of reaction is the reciprocal/inverse of time ie. rate 1/time
Note: Rate and time are inversely related i.e. a smaller time is a higher rate, a longer time is a slower rate
Explain the significance of using the same shape/size of conical flask during each run
• Only one variable can be changed in order for the experiment to be a fair test i.e. the concentration of sodium thiosulfate
• All other possible variables must remain fixed
Note:
- A wider conical flask will take a longer time to form sufficient sulfur to obscure the cross and the reaction rate would seem slower
- A narrower conical flask will take a shorter time to form sufficient sulfur to obscure the cross and the reaction rate would seem faster