Quantification of Microbial Growth
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
74 Terms
How is microbial growth typically defined?
Microbial growth is typically defined as an increase in the number of cells, usually resulting from binary fission.
How is microbial growth measured in liquid culture?
Growth in liquid culture is measured per volume unit.
Why is an increase in cell number not a suitable measure for filamentous microbes?
Filamentous microbes grow differently, often through hyphal extension or sporulation, so other parameters are used to measure their growth.
What are the main types of microbial division?
Binary fission (most common)
Budding
Special types like hyphal growth (tip growth in filamentous microbes) and sporulation.
What are the two main modes of microbial growth?
Planktonic growth – cells suspended in a liquid (e.g., liquid culture, water, blood, urine).
Sessile growth – cells attached to a surface, commonly forming biofilms or colonies on solid media.
What is generation time (g or td)?
The time required for microbial cells to double in number.
What factors influence the time required for microbial cells to divide?
Genetic, nutritional, and physical factors.
How fast does Escherichia coli double under optimal conditions?
Every 20 minutes.
What are the two methods for culturing microbes in liquid media?
Batch culture and continuous culture.
What is an inoculum?
The initial number of cells added to sterile liquid media to start a microbial culture.
What happens after inoculation in liquid media?
Upon adjustment, the cells begin dividing and growing.
What is the log phase (exponential growth phase) in microbial growth?
The log phase is when the microbial population grows rapidly, and cell numbers double at a fixed growth rate constant within each time interval.
What does the equation N = N0 x 2ⁿ describe in microbial growth?
The equation shows the relationship between the initial number of cells (N0) and the final number of cells (N) after a period of exponential growth, with n representing the number of doublings (generations).
How does cell number change during the exponential growth phase?
The number of cells doubles with each generation (e.g., 1 → 2 → 4 → 8 → 16, etc.).
Why is a logarithmic y-axis useful when graphing microbial growth?
Exponential growth appears as a straight line when plotted on a log scale.
Makes it easier to identify the period of exponential growth.
Often visualised using semilogarithmic graphing paper.
What equation describes the doubling time (td) of an exponentially growing population?
td = Δt / n or td = (t - t₀) / n
Also written as g = t / n (in Brock).
Δt = Duration of exponential growth.
n = Number of generations during that period.
What do t and t₀ represent in the doubling time equation?
t = End point of exponential growth.
t₀ = Start point of exponential growth.
The selected time interval does not have to be the exact start and end of log growth.
What factors influence microbial doubling time (td)?
Chemical and physical environment, such as:
Nutrients
Temperature
pH
Doubling time is not a fixed characteristic of a single organism—it varies with conditions.
How does faster mean generation time (g) affect the growth curve on a log scale?
A faster mean generation time results in a steeper slope of the straight line in the log-scaled growth curve
What is the equation for the slope of a microbial growth curve?
Slope = log₁₀(N - N₀) / Δt
N = Final cell number
N₀ = Initial cell number
Δt = Duration of exponential growth
How does doubling time affect the length of the log phase?
If cultures can reach the same maximum cell number, the log phase will be longer for the culture with the slower doubling time
Why is it difficult to directly measure the number of generations in microbial growth?
The number of generations is not easy to measure directly.
Cell numbers are generally easier to measure in the lab.
What equation is typically used to determine the doubling time (td) of microbial growth?
td = 0.301 × (t - t₀) / log₁₀(N / N₀)
t = End time of exponential growth
t₀ = Start time of exponential growth
N₀ = Initial cell number
N = Final cell number
What does Equation 3 describe in terms of microbial growth?
Equation 3 describes the relationship between the time interval of exponential growth (Δt), the starting number of cells (N₀), and the cell number (N) after exponential growth to calculate the doubling time (td).
What does the growth rate (μ) represent in microbial growth?
μ defines the rate of population growth at any time point.
Its unit is per time interval, such as h⁻¹ or min⁻¹.
How is μ related to instantaneous growth rate in microbial growth?
Brock refers to μ as the instantaneous growth rate constant, abbreviated as k.
What is true about the growth rate (μ) during the log phase of microbial growth?
μ remains constant during the log phase of a culture
Why is the growth rate (μ) a practical measure in microbiology?
μ allows meaningful comparison of microbes, such as different strains, variants, or under varying growth conditions.
What is μmax in terms of microbial growth?
μmax is the maximum growth rate a microbe can achieve under optimal environmental conditions (e.g., nutrients, temperature, pH, aeration)
What equation describes the relationship between growth rate (μ) and cell numbers during exponential growth?
μ = 2.303 × log₁₀(N / N₀) / (t - t₀)
N = Final cell number
N₀ = Initial cell number
Δt (t - t₀) = Duration of exponential growth
What does Equation 4 describe in terms of microbial growth?
Equation 4 describes the fixed relationship between growth rate (μ) and cell numbers at the start (N₀) and end (N) of exponential growth, and the selected time interval (Δt).
μ = 2.303 × log₁₀(N / N₀) / (t - t₀)
How is growth rate (μ) related to doubling time (td)?
μ can be calculated directly from the doubling time (td), and vice versa.
μ = ln2 / td (or μ = 0.693 / td).
td = 0.693 / μ.
What is the difference between Δt and the doubling time (td)?
Δt is the time interval for exponential growth (not the doubling time).
Doubling time (td) specifically refers to the time it takes for the cell number to double.
What is doubling time (td) in microbial growth?
Doubling time (td) is also known as generation time (g).
It is the time required for the microbial population to double in number.
What does growth rate (μ) define in microbial growth?
Growth rate (μ) defines the rate of population growth at any specific time point.
Its unit is typically per time interval, such as h⁻¹ or min⁻¹.
How is μ related to the log phase of microbial growth?
μ is constant during the log phase of a culture.
What is batch culture in microbial growth?
Batch culture is a closed-system microbial culture with a fixed volume
What are the four phases typically seen in the growth curve of batch cultures?
Lag phase
Log (exponential) phase (doubling at fixed growth rate μ)
Stationary phase
Decline (death) phase
How does gene expression change during microbial growth phases in batch culture?
Extensive changes in gene expression occur during the adaptation to different growth phases.
These changes result in massive fluctuations in growth rates.
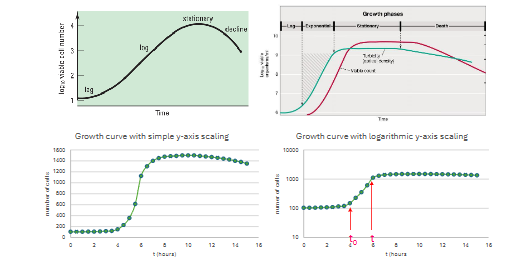
Why are growth curves for batch cultures not always "clear" when determining growth parameters?
Growth curves can show four phases, but they may not be distinct.
To compute μ or td in the log phase, you need to set the t and t₀ on the graph.
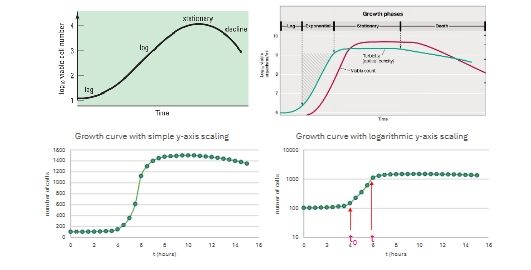
How can you make it easier to determine μ or td in the log phase of a growth curve?
It's easier to use a semi-logarithmic scale (or graph log₁₀ cell numbers) to set t and t₀ for accurate measurement
What are the key considerations for culturing and quantifying microbes?
Appropriate culture medium with proper nutritional requirements.
Maintain a pure 'axenic' culture using aseptic techniques.
Counting cell numbers: Total cell count and viable cell count.
Use practical proxies for cell density, such as turbidity or biomass.
What are the different types of culture media used in microbial growth?
Defined media: Exact chemical composition is known.
Complex media: Composed of microbial, animal, or plant digests (e.g., yeast or meat extracts).
Enriched media: Complex media plus highly nutritious materials (e.g., blood serum or milk) for fastidious microbes.
Selective media: Contain compounds that selectively inhibit the growth of some microbes.
Differential media: Contain an indicator, usually a dye, to detect particular metabolic reactions during growth.
What is total cell count and how is it measured using light microscopy?
Total cell count involves observing and enumerating ALL cells present in a known volume of liquid samples using light microscopy.
How does a bacterial counting chamber work for total cell count?
A bacterial counting chamber has a grid with squares etched on a surface and a fixed volume.
Different grid types include Helber, Thoma, Improved Neubauer, and Petroff-Hauser.
What is the typical depth of a bacterial counting chamber?
The typical depth of a bacterial counting chamber is 0.01 or 0.02 mm
How do haemocytometers for eukaryotic cells differ from bacterial counting chambers?
Haemocytometers for eukaryotic cells are deeper, typically 0.1 or 0.2 mm (Improved Neubauer grid)
What are some limitations of using counting chambers for total cell count?
Counting both live and dead cells.
The need for dilution to avoid too many or too few cells for accurate counting
What does viable cell count measure in microbial growth?
Viable cell count measures the living, reproducing population of microbes.
It assumes each viable cell will form one colony.
What is the most common method for viable cell count?
The most common method for viable cell count is the spread-plate method
How is a sample typically prepared for viable cell count?
The sample to be counted is usually diluted in 10-fold steps
What is the ideal number of colonies on a plate for reproducible and accurate viable cell count results?
30–300 colonies should be counted on the plate for reliable results
How are results of viable cell counts measured?
Results are measured as colony-forming units (CFU)
How is cell density typically reported in microbial growth studies?
Cell density is usually reported as cell number or colony-forming units (CFU) per milliliter (ml⁻¹).
What should be ensured when graphing or comparing microbial growth curves?
Always compare or graph either cell numbers/CFU OR cell density—do not mix the two.
When are viable counts particularly useful?
Viable counts are reliable for counting specific microbes on selective media.
Common applications include microbial detection in food or sewage
How does CFU compare to total cell count during active growth in batch culture?
CFU is typically lower than the total cell count in active growth phases
What is the viable count anomaly, and what factors contribute to it?
Viable count anomaly occurs due to:
Different organisms in a mixture having different growth characteristics.
Presence of Viable But Not Culturable (VBNC) cells.
Dead cells being counted in the total cell count but not in CFU.
How is cell density often represented in microbial growth curves?
Cell density is often shown as Optical Density (OD) vs. time in growth curves.
What is Optical Density (OD) in microbial growth studies?
Optical Density (OD), also known as Absorbance or turbidity, is a measure of how much light is scattered by cells in a liquid culture
How is OD measured, and what is it used for?
OD is measured as turbidity using a spectrophotometer.
It serves as a proxy for cell density in microbial cultures.
At what wavelength is OD commonly measured?
OD is commonly measured at 600 nm (OD600)
How is Optical Density (OD) related to cell number for unicellular organisms?
Optical Density (OD) is directly proportional to the cell number for unicellular organisms. As the cell density increases, the OD also increases because more cells scatter light, making the solution appear more turbid. This relationship is often used to estimate cell concentration in culture, with higher OD values indicating a greater number of cells.
Why is a standard curve needed to relate OD to cell count?
Lambert-Beer’s law does not apply to microbial cultures.
A standard curve must be established to accurately relate direct or viable cell counts to turbidity (OD) values.
Why does OD underestimate cell density at high values?
At high OD, light scattering leads to an underestimation of actual cell density.
It is better to dilute the culture and then calculate the real OD
Why is OD often a poor proxy for multicellular organisms or clumping cells?
OD is not always proportional to cell density for multicellular or clumped cells
What alternative proxies can be used for cell density when OD is not reliable?
Biomass measurements, such as:
Wet weight or dry weight of pelleted cells.
DNA concentration (µg DNA per ml culture) – assumes constant DNA per cell.
Total protein concentration (mg protein per ml culture) – assumes constant protein per cell.
These methods are more laborious but proportional to cell density.
What is a continuous culture, and how does it differ from a batch culture?
Continuous culture is an open-system microbial culture of fixed volume, unlike batch culture, which is a closed system
What is a chemostat, and why is it used in continuous culture?
A chemostat is the most common continuous culture device.
It maintains a steady state, where:
Cell density remains constant over time.
Substrate concentration does not change over time.
The culture remains in the same growth phase, with no transitions.
How can growth rate and population density be controlled in a chemostat?
Growth rate (μ) is controlled by the dilution rate (D), which depends on:
The flow rate of incoming medium and effluent.
The vessel volume.
Cell density is controlled by the concentration of a limiting nutrient in the medium.
How can growth rate and population density be controlled independently in a chemostat?
Growth rate (μ) or doubling time (td) is controlled by the dilution rate (D) (flow rate of inlet/effluent & vessel volume).
Cell density is determined by the concentration of a limiting nutrient in the medium.
What are the key steady-state relationships in a chemostat?
Cell density and substrate concentration remain constant over time.
The growth phase stays the same, without transitions
What are the experimental uses of a chemostat?
Can maintain exponential growth for weeks/months.
Used to study:
Microbial physiology
Microbial ecology & evolution
Enrichment & isolation of bacteria from nature.
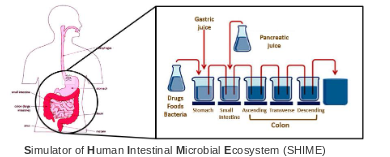
How does the Simulator of Human Intestinal Microbial Ecosystem (SHIME) relate to continuous culture?
Humans and animals naturally maintain their own continuous culture in the gut.
Diarrhea occurs when the effluent rate (outflow) is faster than the inflow rate.
SHIME (Simulator of Human Intestinal Microbial Ecosystem) uses a series of chemostats to mimic the human GI tract, allowing researchers to study gut microbiota dynamics.